Question: Process 1 Process 2 T T2 In the two processes shown in the figure aside, the same amount of heat, Q, is supplied to equal
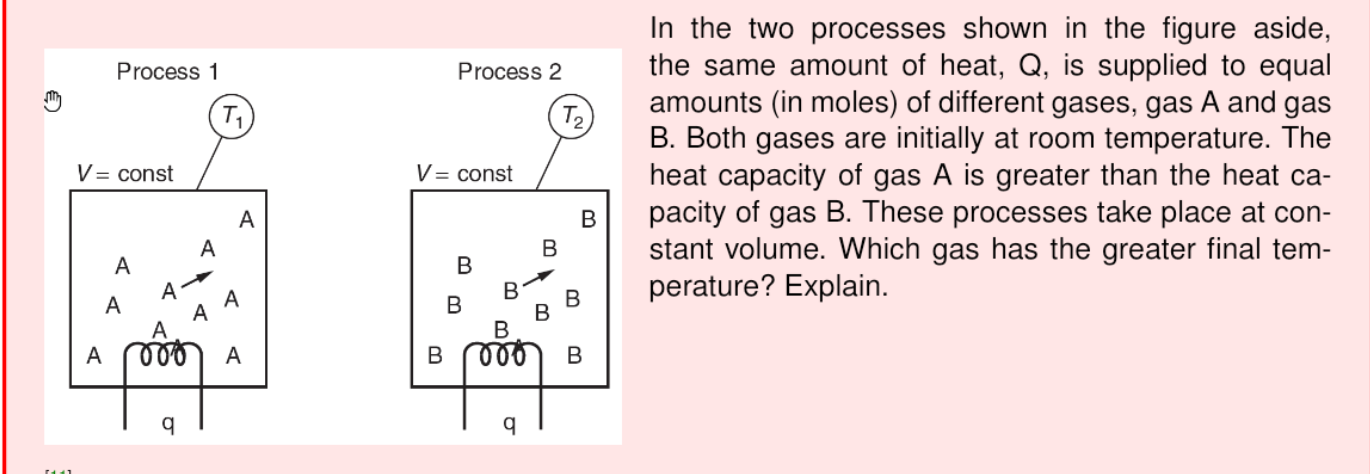
Process 1 Process 2 T T2 In the two processes shown in the figure aside, the same amount of heat, Q, is supplied to equal amounts (in moles) of different gases, gas A and gas B. Both gases are initially at room temperature. The heat capacity of gas A is greater than the heat ca- pacity of gas B. These processes take place at con- stant volume. Which gas has the greater final tem- perature? Explain. V = const V= const A B B B A A . B B B 000 A 000 A B 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


