Question: How do you get the ln VP values and 1/T values? Thanks! 1. The vapor pressure of an unknown liquid was measured as described in
How do you get the ln VP values and 1/T values? Thanks!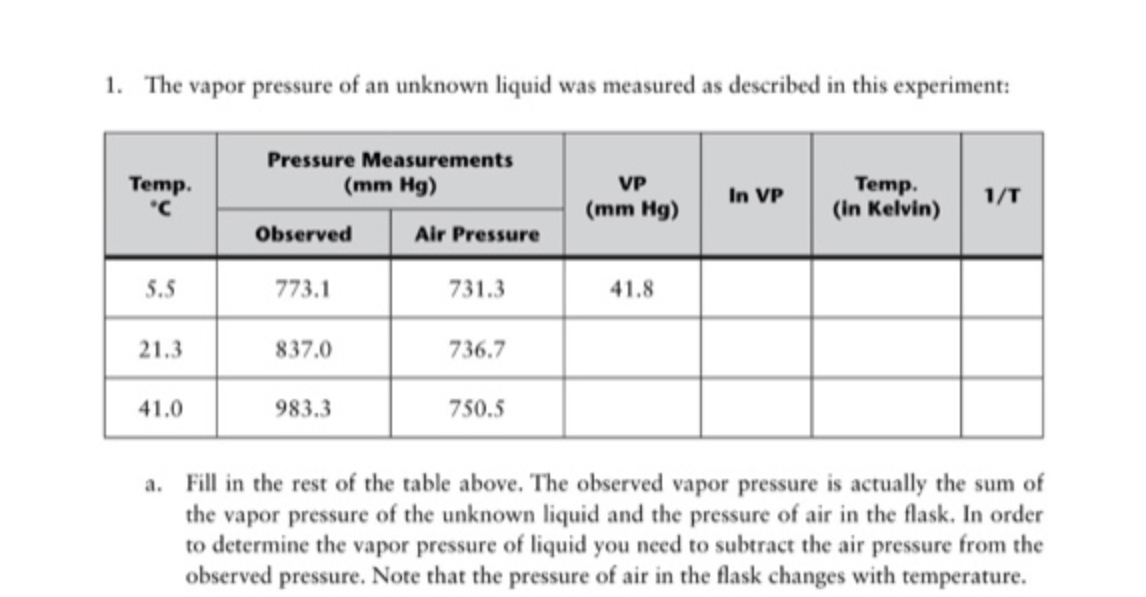
1. The vapor pressure of an unknown liquid was measured as described in this experiment: a. Fill in the rest of the table above. The observed vapor pressure is actually the sum of the vapor pressure of the unknown liquid and the pressure of air in the flask. In order to determine the vapor pressure of liquid you need to subtract the air pressure from the observed pressure. Note that the pressure of air in the flask changes with temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


