Question: how do you solve this step by step please 1. Consider the following reaction 2CO(g)+O2(g)2CO2(g) a. What is the average rate for the reaction if
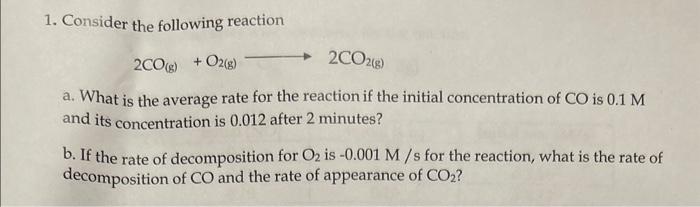
1. Consider the following reaction 2CO(g)+O2(g)2CO2(g) a. What is the average rate for the reaction if the initial concentration of CO is 0.1M and its concentration is 0.012 after 2 minutes? b. If the rate of decomposition for O2 is 0.001M/s for the reaction, what is the rate of decomposition of CO and the rate of appearance of CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


