Question: How much work, w, must be done on a system to decrease its volume from 15.0 L to 6.0L by exerting a constant pressure of
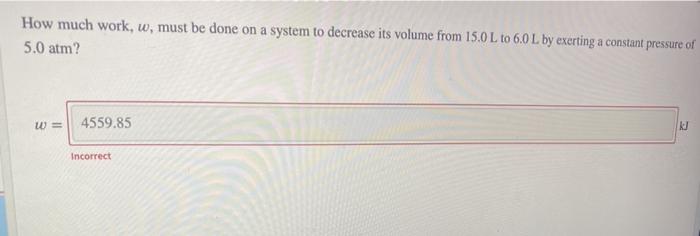
How much work, w, must be done on a system to decrease its volume from 15.0 L to 6.0L by exerting a constant pressure of 5.0 atm? w= 4559.85 Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


