Question: how to find enthlapy and jules evolved? (-1 pts) Incorrect. Use the equation H=molossallqm, keeping in mind that qrxn=qcalorimeter- (-1 pts) Incorrect. Assuming that the
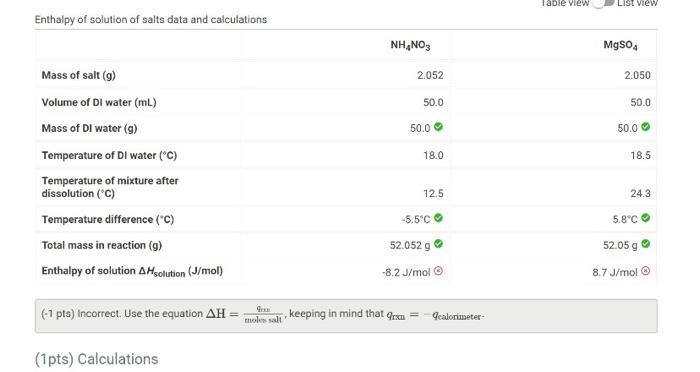
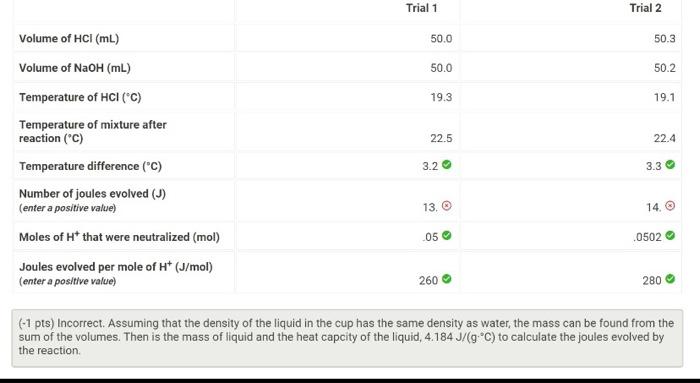
(-1 pts) Incorrect. Use the equation H=molossallqm, keeping in mind that qrxn=qcalorimeter- (-1 pts) Incorrect. Assuming that the density of the liquid in the cup has the same density as water, the mass can be found from the sum of the volumes. Then is the mass of liquid and the heat capcity of the liquid, 4.184J/(gC) to calculate the joules evolved by the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


