Question: HSO3 is a strong acid weak acid strong base weak base neither acid nor base QUESTION 8 Calcium hydroxide is a strong acid weak
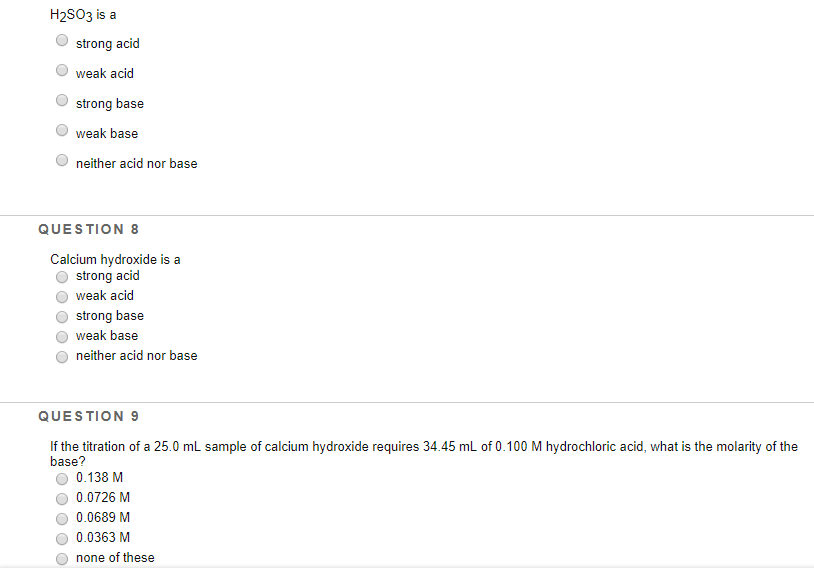
HSO3 is a strong acid weak acid strong base weak base neither acid nor base QUESTION 8 Calcium hydroxide is a strong acid weak acid strong base weak base neither acid nor base QUESTION 9 If the titration of a 25.0 mL sample of calcium hydroxide requires 34.45 mL of 0.100 M hydrochloric acid, what is the molarity of the base? 0.138 M 0.0726 M 0.0689 M O 0.0363 M none of these
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
Answers Question 7 HSO3 is a strong acid This is because it dissociates Fully in water Question ... View full answer

Get step-by-step solutions from verified subject matter experts


