Question: html code for website (one page only ) exactly as shown below Fact 1 Water (H2O) is a polar inorganic compound that is at room
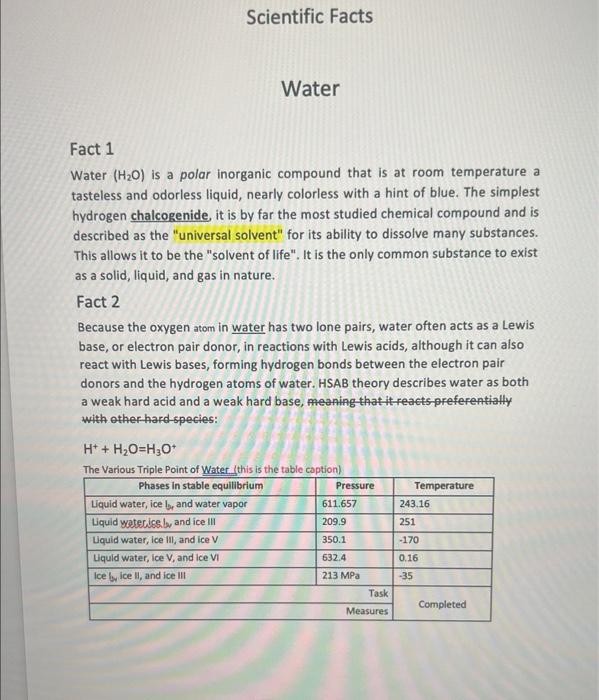

Fact 1 Water (H2O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, nearly colorless with a hint of blue. The simplest hydrogen chalcogenide, it is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances. This allows it to be the "solvent of life". It is the only common substance to exist as a solid, liquid, and gas in nature. Fact 2 Because the oxygen atom in water has two lone pairs, water often acts as a Lewis base, or electron pair donor, in reactions with Lewis acids, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory describes water as both a weak hard acid and a weak hard base, meaning that it reaets preferentially with other hard-species: H++H2O=H3O+ The Various Triole Point of Water Ithis is the table coption] Article Contents 1. Chemical and physical properties 2. Taste and odor 3. Distribution in nature 3.1 In the universe 3.1.1 Water vapor 3.1.2 Liquid water 3.1.3 Water ice 3.1.4 Exotic forms 3.2 Water and habitable zone 4. On Earth 4.1 Water cycle. 4.2 Fresh water storage. 4.3 Sea water and tides. Reader's Comments: Name: Department: Cnmmante
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


