Question: I am currently trying to understand the binary diffusion according to Onsager and to work it up for our term paper. There is one point
I am currently trying to understand the binary diffusion according to Onsager and to work it up for our term paper. There is one point where I am stuck:
Our task is to write down the individual mathematical steps to get from Eq. (1) to Eq. (2) to gain a mathematical understanding about binary diffusion systems.
Given is the following formula:
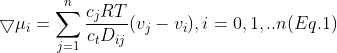
where 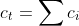 and
and 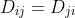 are transport parameters of dimension from the diffusion coefficient characterizing the interactions between species
are transport parameters of dimension from the diffusion coefficient characterizing the interactions between species 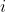 and
and 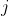 . They are defined only for ij and are independent of the coordinate system to which the velocities
. They are defined only for ij and are independent of the coordinate system to which the velocities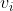 are referred, since the equation concerns only the differences of the velocities. In the following, we will consider a system containing three species: the solvent (i = 0), species one (i = 1) and species two (i = 2). Since the electrochemical potentials satisfy the relation
are referred, since the equation concerns only the differences of the velocities. In the following, we will consider a system containing three species: the solvent (i = 0), species one (i = 1) and species two (i = 2). Since the electrochemical potentials satisfy the relation 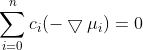 , the solution of Eq. 1 can be written in the following form:
, the solution of Eq. 1 can be written in the following form:
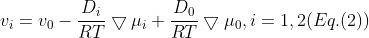
where the effective diffusion coefficients 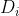 are defined as follows:
are defined as follows:
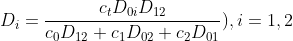
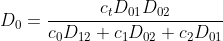
Since the system of equations is linearly dependent on the given equation, one equation must be deleted, e.g. for i = 0. Then only two equations remain, from which the velocities v1 and v2 can be calculated.
Thank you for the effort and help and time.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


