Question: I am not sure on how to get the answer for B What is the binding free energy for the formation of the complex ED?
I am not sure on how to get the answer for B
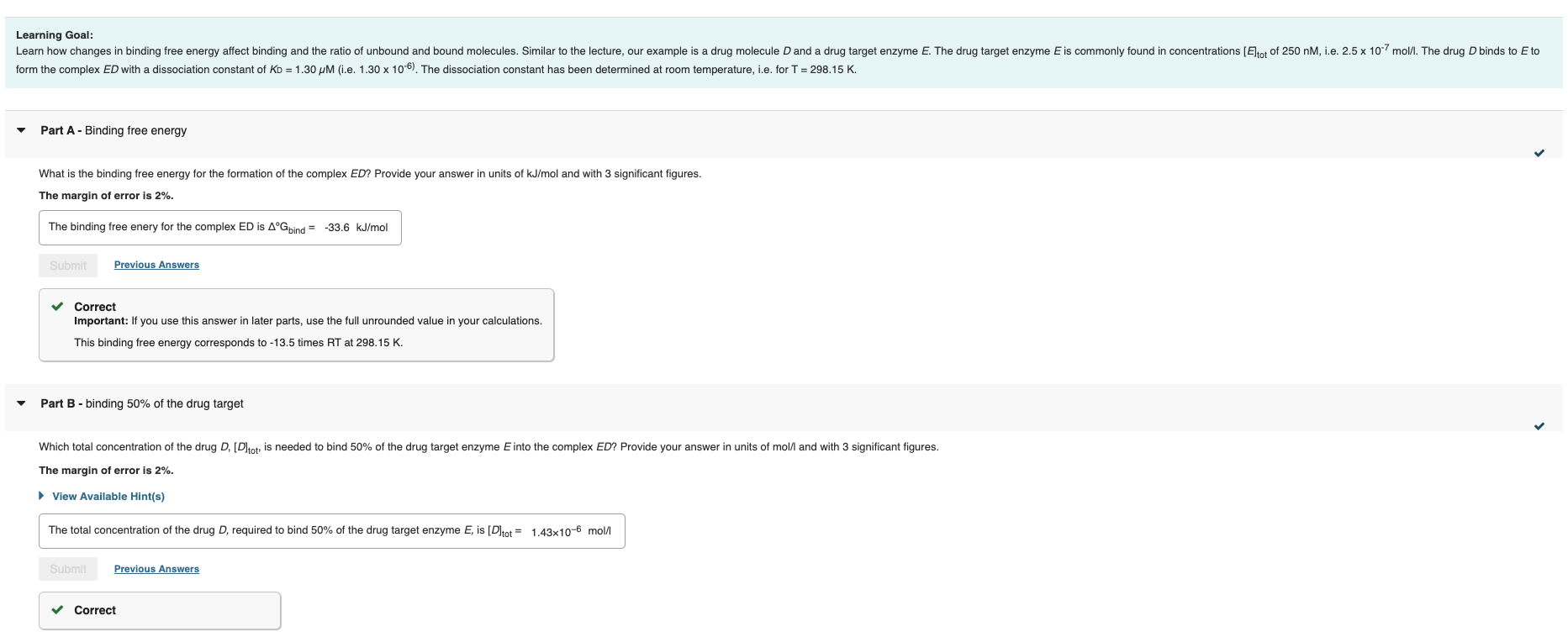
What is the binding free energy for the formation of the complex ED? Provide your answer in units of kJ/mol and with 3 significant figures. The margin of error is 2%. The binding free enery for the complex ED is Gbind=33.6kJ/mol Correct Important: If you use this answer in later parts, use the full unrounded value in your calculations. This binding free energy corresponds to 13.5 times RT at 298.15K. Part B - binding 50% of the drug target Which total concentration of the drug D,[D]tot, is needed to bind 50% of the drug target enzyme E into the complex ED ? Provide your answer in units of mol/ and with 3 significant figures. The margin of error is 2%. View Available Hint(s) The total concentration of the drug D, required to bind 50% of the drug target enzyme E, is [D]tot=1.43106mol//
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


