Question: I am really lost please help me understand this from the begining. The reaction of butyl-bromide (CH3)3CBr with water is represented by the equation: (CH3)3CBr+H2O(CH3)3COH+HBr
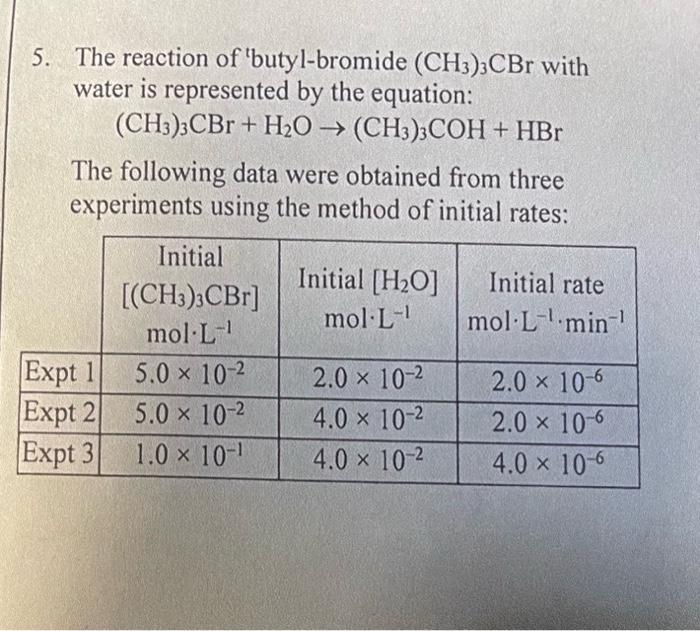

The reaction of "butyl-bromide (CH3)3CBr with water is represented by the equation: (CH3)3CBr+H2O(CH3)3COH+HBr The following data were obtained from three experiments using the method of initial rates: a. What is the order with respect to (CH3)3CBr ? b. What is the order with respect to H2O ? c. What is the overall order of the reaction? d. Write the rate law. e. Calculate the rate constant, k, for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


