Question: really lost, please provide reasoning 2. In order to study the photochemical decay of aqueous bromine in bright sunlight, a small quantity of liquid bromine
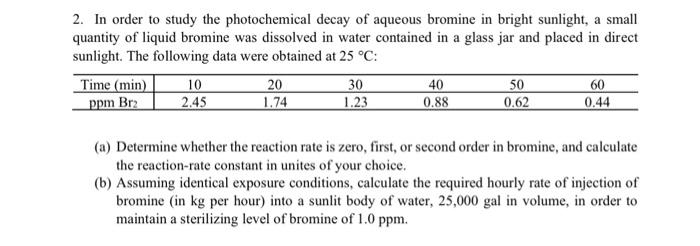
2. In order to study the photochemical decay of aqueous bromine in bright sunlight, a small quantity of liquid bromine was dissolved in water contained in a glass jar and placed in direct sunlight. The following data were obtained at 25C : (a) Determine whether the reaction rate is zero, first, or second order in bromine, and calculate the reaction-rate constant in unites of your choice. (b) Assuming identical exposure conditions, calculate the required hourly rate of injection of bromine (in kg per hour) into a sunlit body of water, 25,000 gal in volume, in order to maintain a sterilizing level of bromine of 1.0ppm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


