Question: I can't figure this question out, please explain! 2. A mixture of 1.0 mole carbon dioxide and 1.0 mole carbon monoxide are contained in a
I can't figure this question out, please explain!
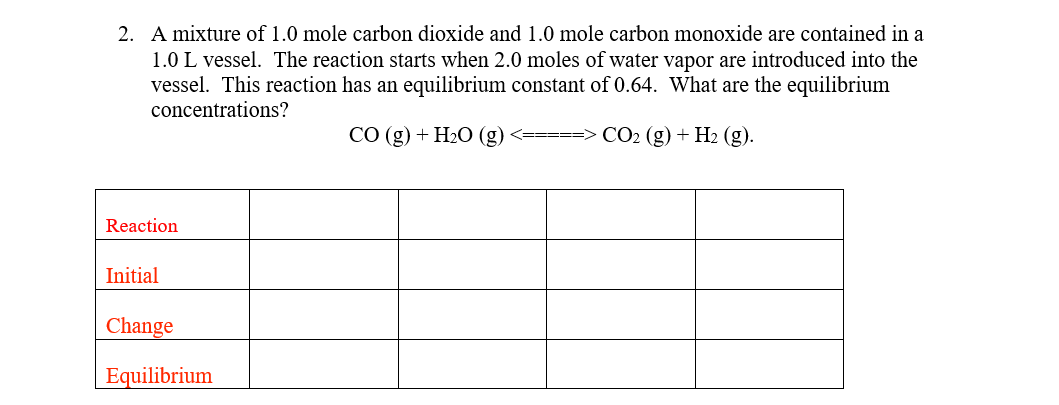
2. A mixture of 1.0 mole carbon dioxide and 1.0 mole carbon monoxide are contained in a 1.0L vessel. The reaction starts when 2.0 moles of water vapor are introduced into the vessel. This reaction has an equilibrium constant of 0.64. What are the equilibrium concentrations? CO(g)+H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


