Question: I Consider a reaction at a given temperature with A = 3.4 A 10 For this reaction you would expect that at equilibrium, the concentration
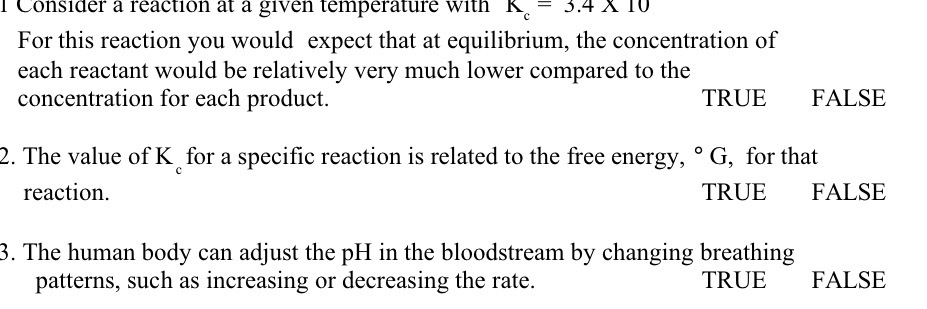
I Consider a reaction at a given temperature with A = 3.4 A 10 For this reaction you would expect that at equilibrium, the concentration of each reactant would be relatively very much lower compared to the concentration for each product. TRUE FALSE 2. The value of K for a specific reaction is related to the free energy, . G, for that reaction. TRUE FALSE 3. The human body can adjust the pH in the bloodstream by changing breathing patterns, such as increasing or decreasing the rate. TRUE FALSE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


