Question: I do not know how to write the answer into the box. I know the answer is that there is no rate of change, and
I do not know how to write the answer into the box. I know the answer is that there is no rate of change, and I even tried entering zero. Help please...
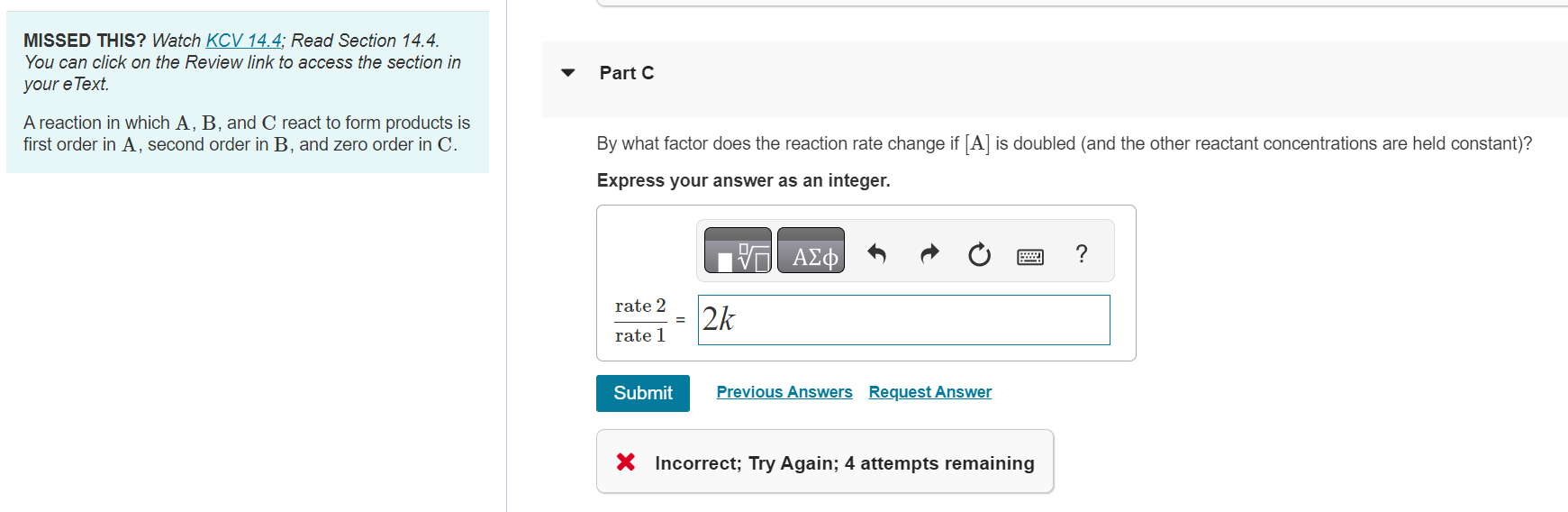
MISSED THIS? Watch KCV 14.4; Read Section 14.4. You can click on the Review link to access the section in your eText. Part C A reaction in which A,B, and C react to form products is first order in A, second order in B, and zero order in C. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? Express your answer as an integer. X Incorrect; Try Again; 4 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


