Question: i dont understand these please help Select the lowest energy conformer of cis-1-t-butyl-4-methylcyclohexane. A) B) C) C Which conformation is more stable? A) A B)
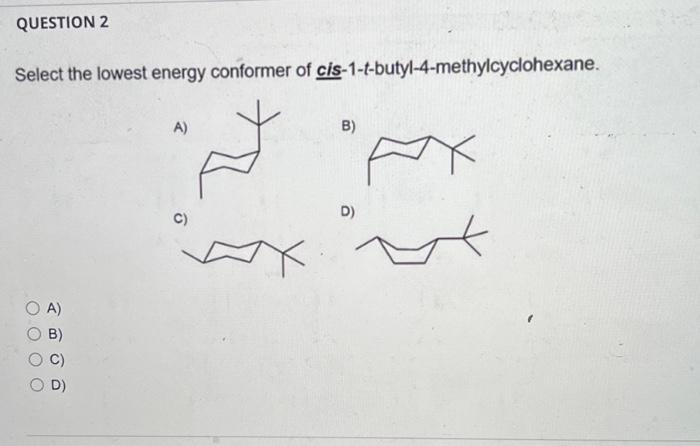
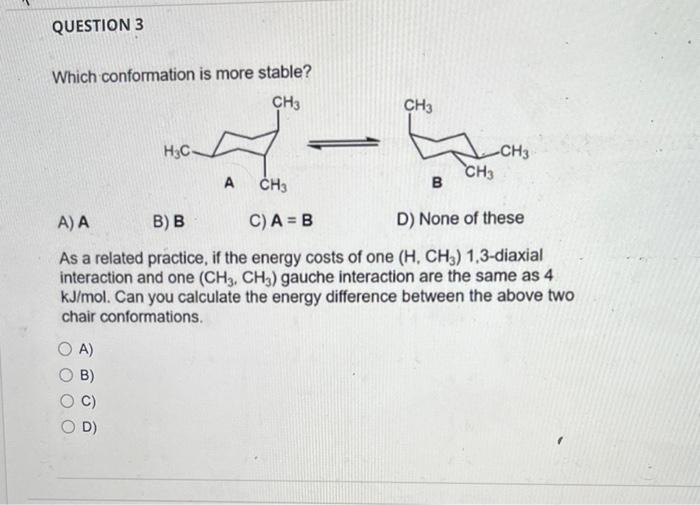
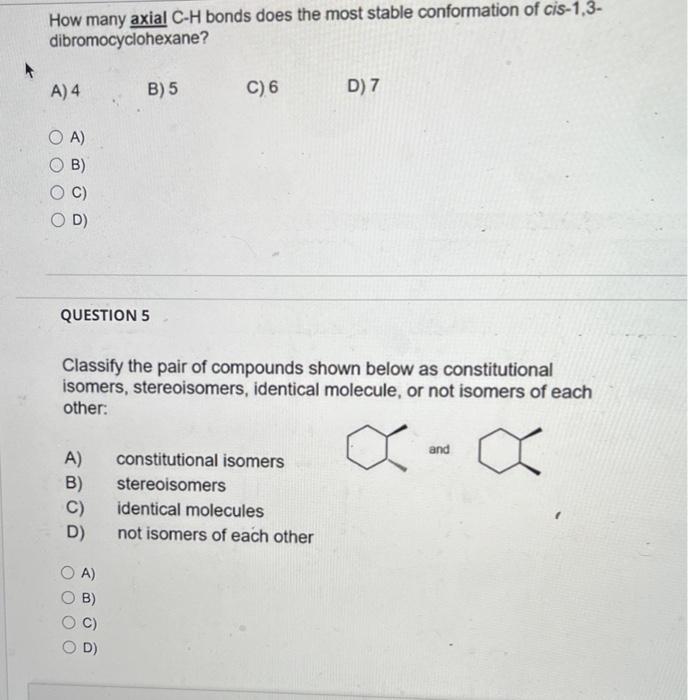
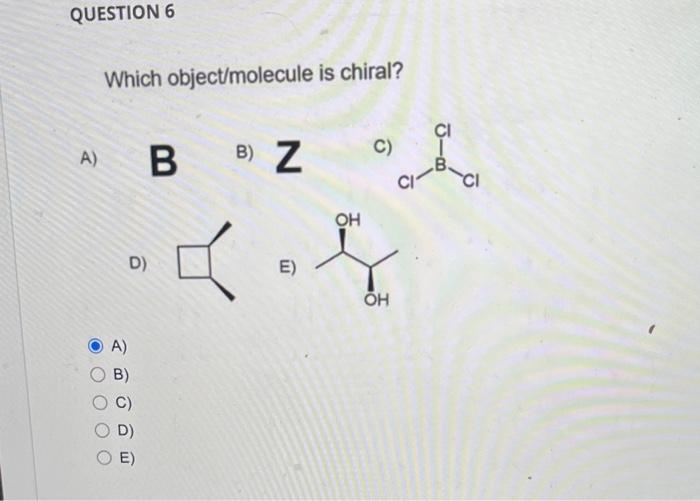
Select the lowest energy conformer of cis-1-t-butyl-4-methylcyclohexane. A) B) C) C Which conformation is more stable? A) A B) B C) A=B D) None of these As a related practice, if the energy costs of one (H,CH3) 1,3-diaxial interaction and one (CH3,CH3) gauche interaction are the same as 4 kJ/mol. Can you calculate the energy difference between the above two chair conformations. A) B) C) D) How many axial C-H bonds does the most stable conformation of cis-1,3dibromocyclohexane? A) 4 B) 5 C) 6 D) 7 A) B) C) D) QUESTION 5 Classify the pair of compounds shown below as constitutional isomers, stereoisomers, identical molecule, or not isomers of each other: A) constitutional isomers and B) stereoisomers C) identical molecules D) not isomers of each other A) B) C) D) QUESTION 6 Which object/molecule is chiral? A) B) 7 C) D) A) B) C) D) E)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


