Question: I don't undestand this problem In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an
I don't undestand this problem 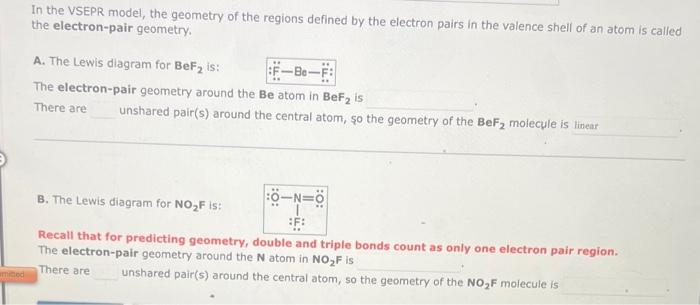

In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry. A. The Lewis diagram for BeF2 is: The electron-pair geometry around the Be atom in BeF2 is There are unshared pair(s) around the central atom, so the geometry of the BeF2 molecule is B. The Lewis diagram for NO2F is: Recall that for predicting geometry, double and triple bonds count as only one electron pair region. The electron-pair geometry around the N atom in NO2F is There are unshared pair(s) around the central atom, so the geometry of the NO2F molecule is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


