Question: Please note that geometry refers to the molecular or lonic geometry. In the VSEPR model, the geometry of the regions defined by the electron pairs
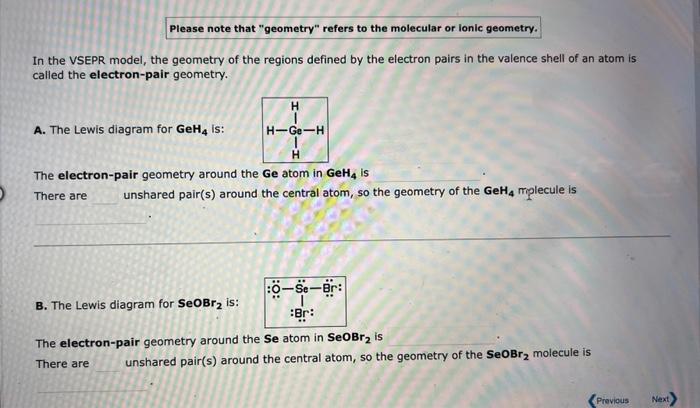
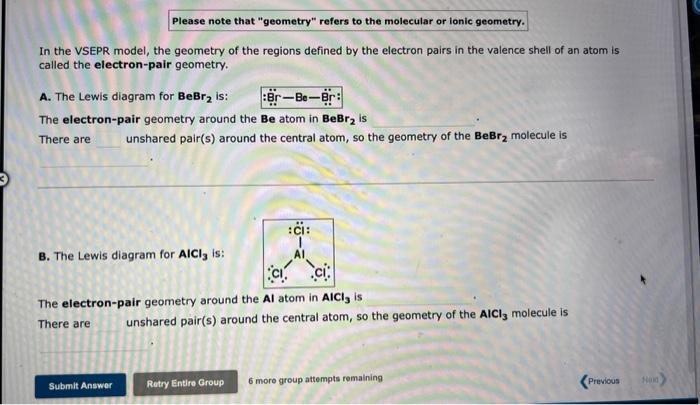
Please note that "geometry" refers to the molecular or lonic geometry. In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry. A. The Lewis diagram for GeH4 is: The electron-pair geometry around the Ge atom in GeH4 is There are unshared pair(s) around the central atom, so the geometry of the GeH4mp1 lecule is B. The Lewis diagram for SeOBr2 is: The electron-pair geometry around the Se atom in SeOBr2 is There are unshared pair(s) around the central atom, so the geometry of the SeOBr2 molecule is Please note that "geometry" refers to the molecular or ionic geometry. In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry. A. The Lewis diagram for BeBr2 is: The electron-pair geometry around the Be atom in BeBr2 is There are unshared pair(s) around the central atom, so the geometry of the BeBr2 molecule is B. The Lewis diagram for AlCl3 is: The electron-pair geometry around the Al atom in AlCl3 is There are unshared pair(s) around the central atom, so the geometry of the AlCl3 molecule is 6 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


