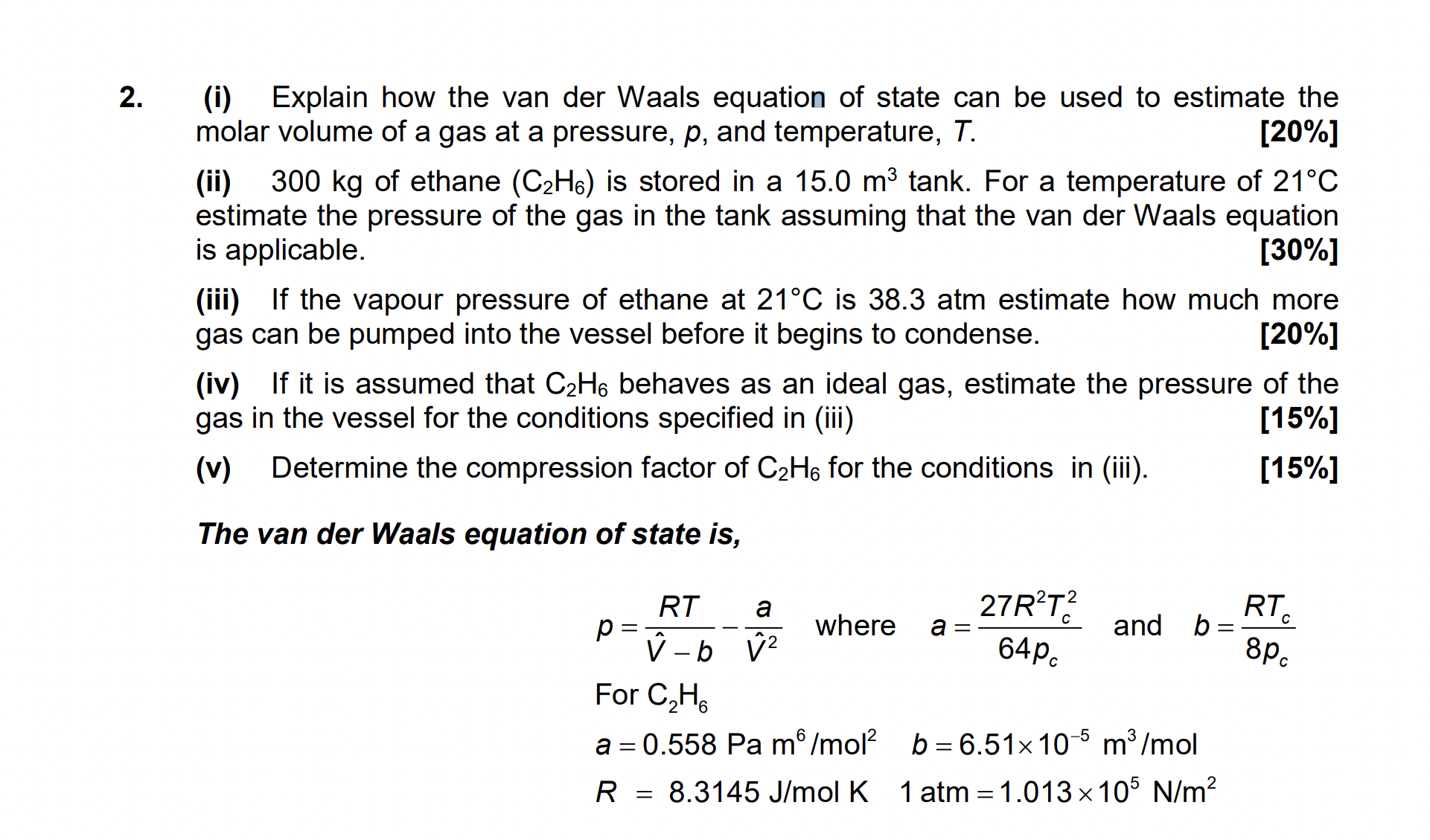
Question: ( i ) Explain how the van der Waals equation of state can be used to estimate the molar volume of a gas at a
i Explain how the van der Waals equation of state can be used to estimate the
molar volume of a gas at a pressure, and temperature,
ii of ethane is stored in a tank For a temperature of
estimate the pressure of the gas in the tank assuming that the van der Waals equation
is applicable.
iii If the vapour pressure of ethane at is atm estimate how much more
gas can be pumped into the vessel before it begins to condense.
iv If it is assumed that behaves as an ideal gas, estimate the pressure of the
gas in the vessel for the conditions specified in iii
v Determine the compression factor of for the conditions in iii
The van der Waals equation of state is
where and
For
olK,atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


