Question: I NEED ABSORPTION AND STRIPPING GRAPHS IN X VS PPM GRAPHS A gas processing plant has an absorber and stripper set up as shown in
I NEED ABSORPTION AND STRIPPING GRAPHS IN X VS PPM GRAPHS
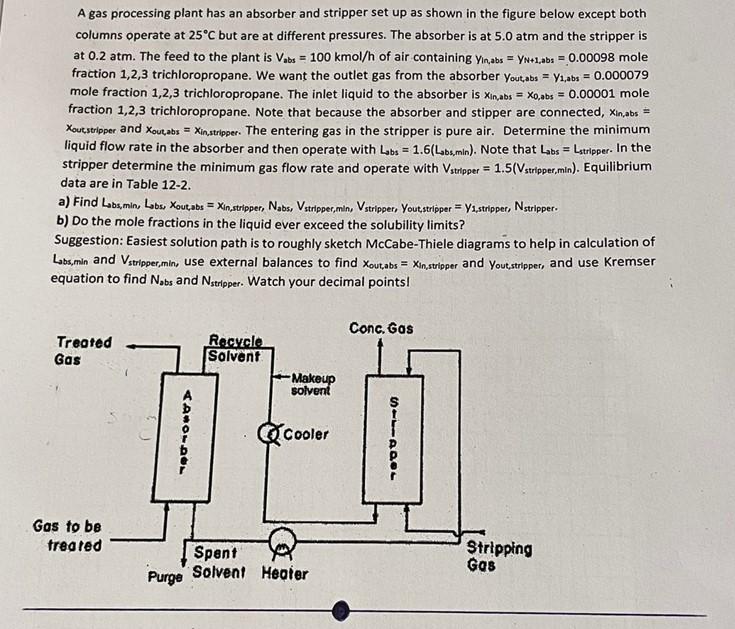
A gas processing plant has an absorber and stripper set up as shown in the figure below except both columns operate at 25C but are at different pressures. The absorber is at 5.0 atm and the stripper is at 0.2 atm. The feed to the plant is Vabs = 100 kmol/h of air containing Yin,abs = YN+1,abs = 0.00098 mole fraction 1,2,3 trichloropropane. We want the outlet gas from the absorber Yout,abs = y1,abs = 0.000079 mole fraction 1,2,3 trichloropropane. The inlet liquid to the absorber is Xin,abs Xo,abs = 0.00001 mole fraction 1,2,3 trichloropropane. Note that because the absorber and stipper are connected, Xin,abs = Xout,stripper and Xout,abs = Xin,stripper. The entering gas in the stripper is pure air. Determine the minimum liquid flow rate in the absorber and then operate with Labs = 1.6(Labs,min). Note that Labs = Lstripper. In the stripper determine the minimum gas flow rate and operate with Vstripper = 1.5(Vstripper,min). Equilibrium data are in Table 12-2. a) Find Labs,min, Labs, Xout,abs = Xin,stripper, Nabs, Vstripper,min, Vstripper, Yout,stripper Y1,stripper, Nstripper. b) Do the mole fractions in the liquid ever exceed the solubility limits? Suggestion: Easiest solution path is to roughly sketch McCabe-Thiele diagrams to help in calculation of Labs,min and Vstripper,min, use external balances to find Xout,abs = Xin,stripper and Yout,stripper, and use Kremser equation to find Nabs and Nstripper. Watch your decimal points! Conc, Gas Treated Recycle Solvent Gas Makeup solvent Gas to be treated Stripping Gas ADBOLDOL Purge Spent Solvent Cooler Heater STT1PQOL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


