Question: I need help answering all the question pleasee. Determine the molecular formula for the compound with a molar mass of 60.10g/mol and the following percent
I need help answering all the question pleasee.
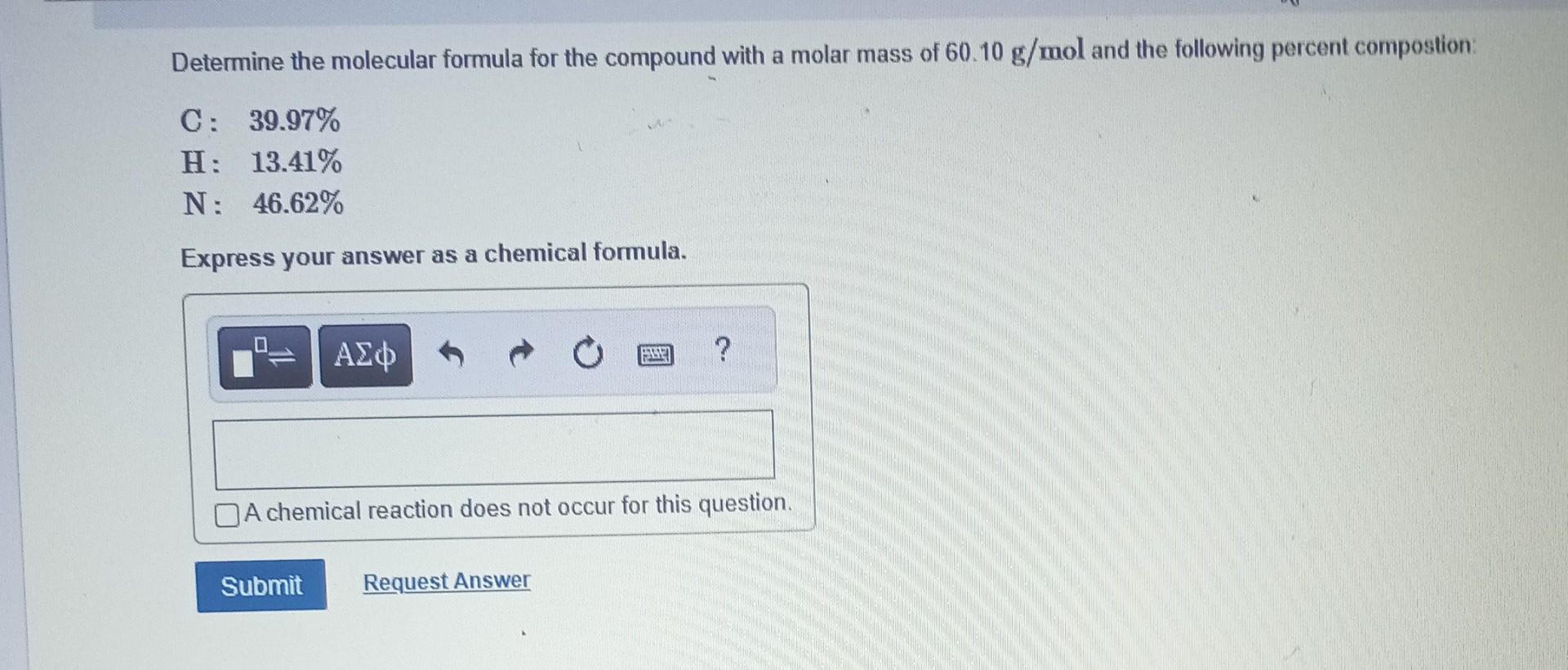
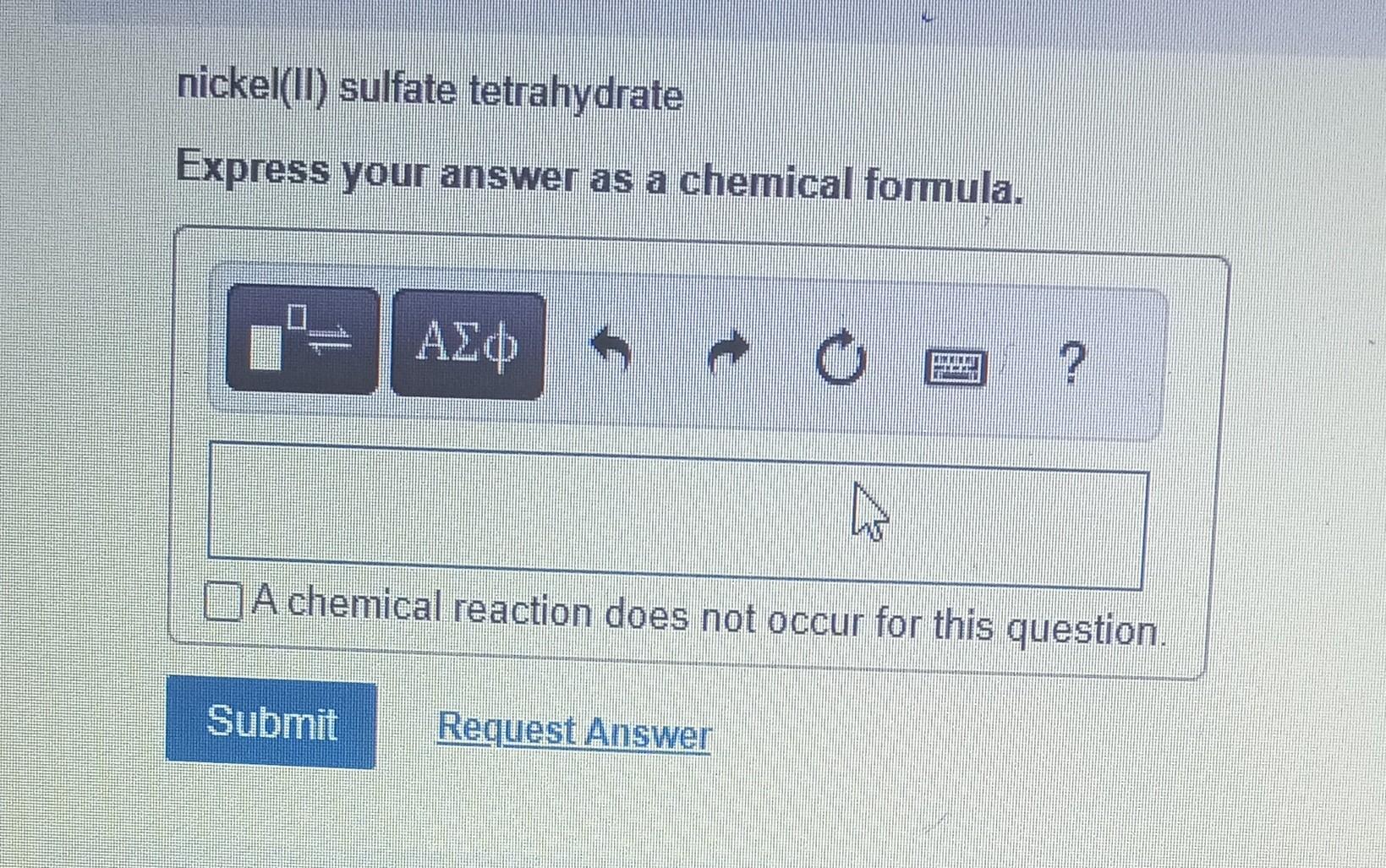
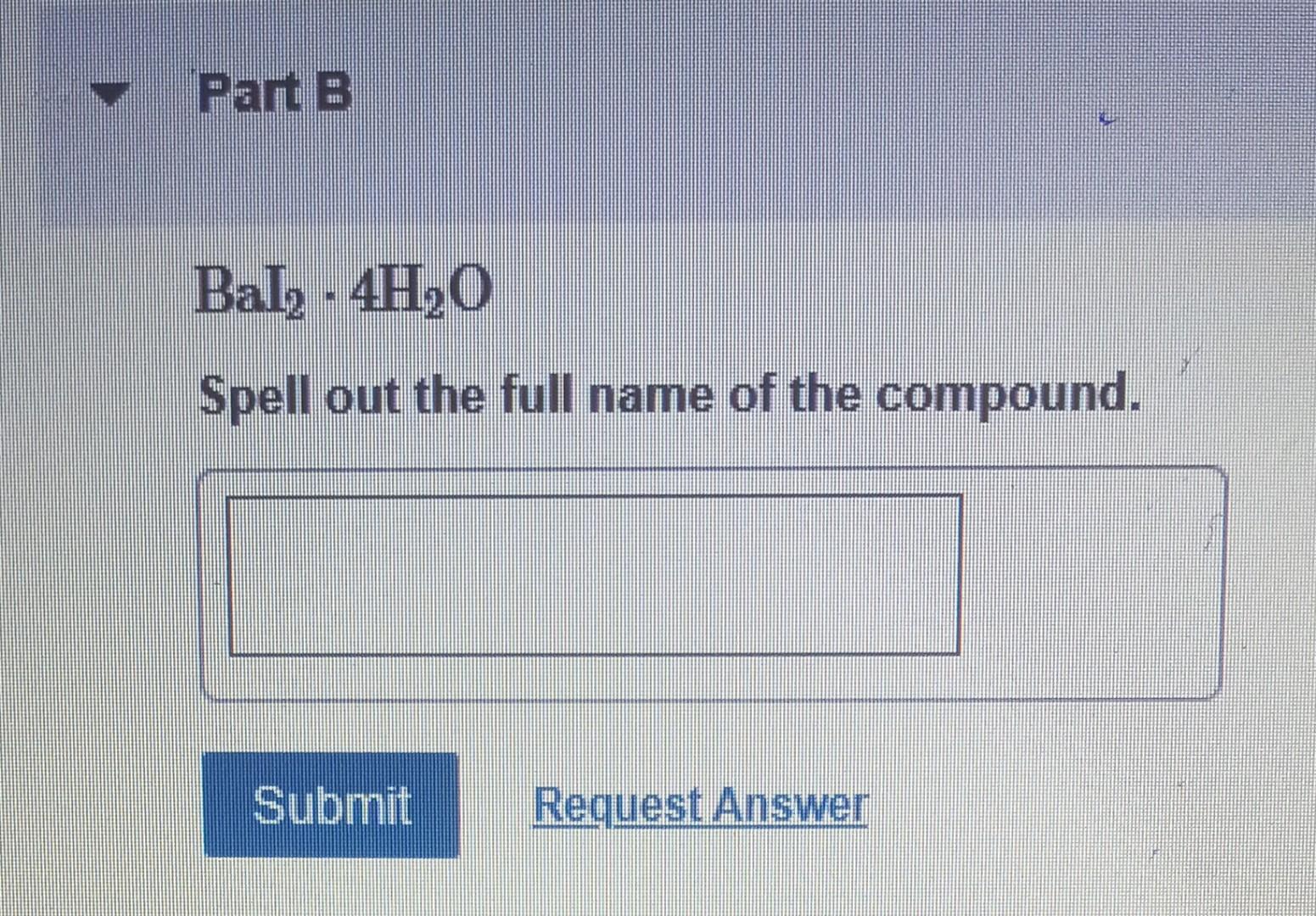
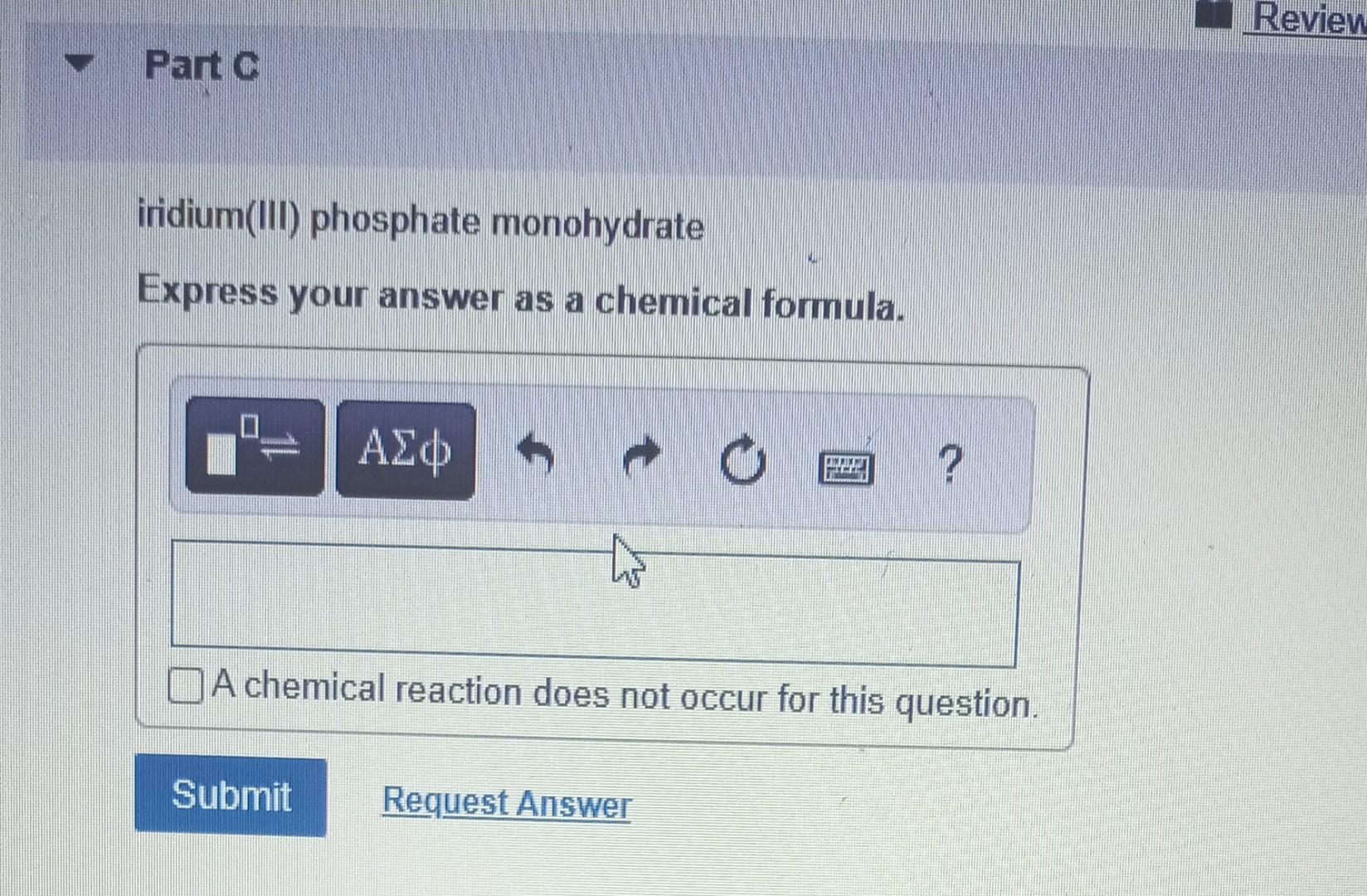
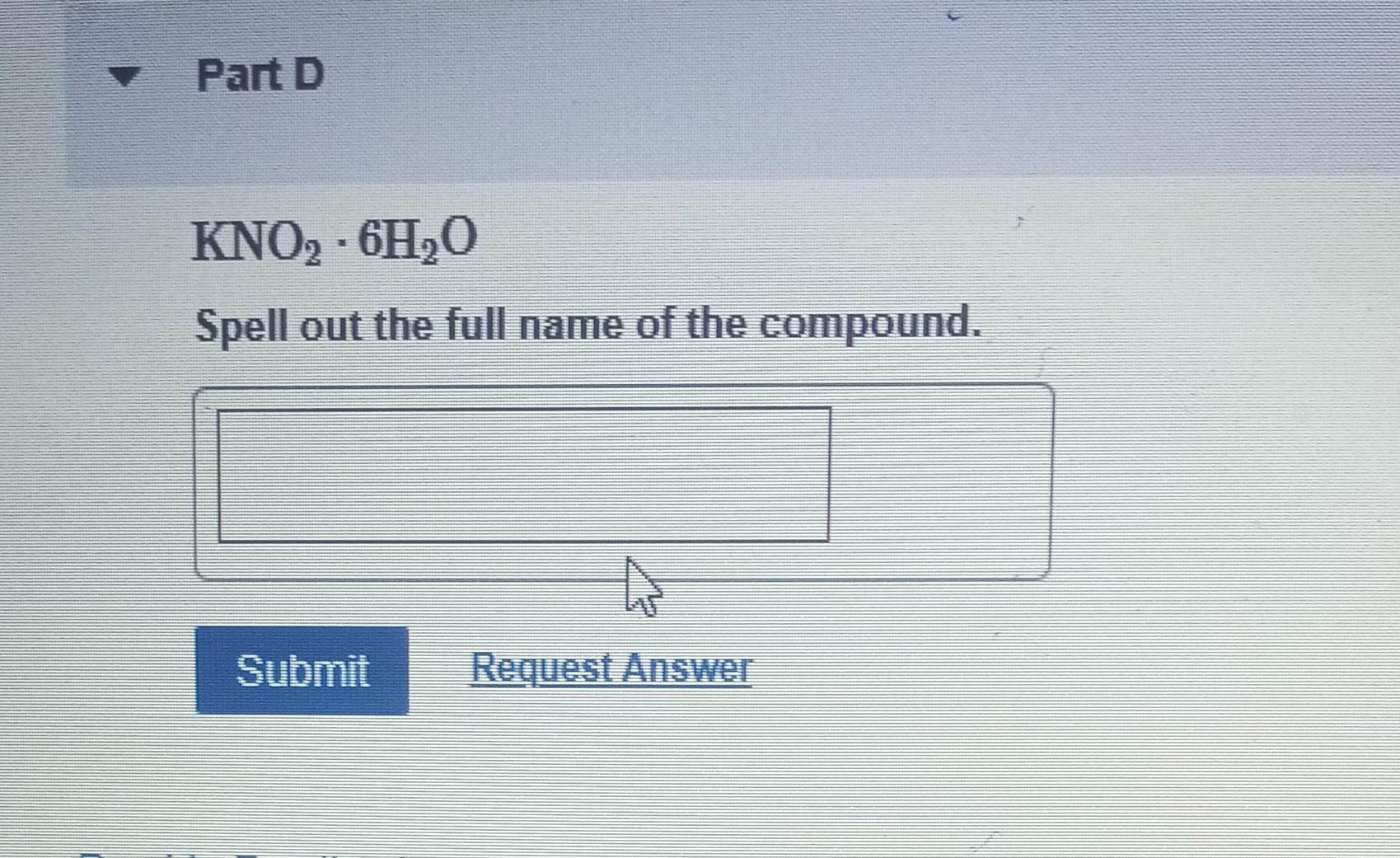
Determine the molecular formula for the compound with a molar mass of 60.10g/mol and the following percent compostion: C:39.97%H:13.41%N:46.62% Express your answer as a chemical formula. nickel(II) sulfate tetrahydrate Express your answer as a chemical formula. Spell out the full name of the compound. iridium(III) phosphate monohydrate Express your answer as a chemical formula. Spell out the full name of the compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


