Question: i need help asap thank you i will rate Select the strongest acid. Consider all resonance patterns and all delocalized electrons in the molecule, which
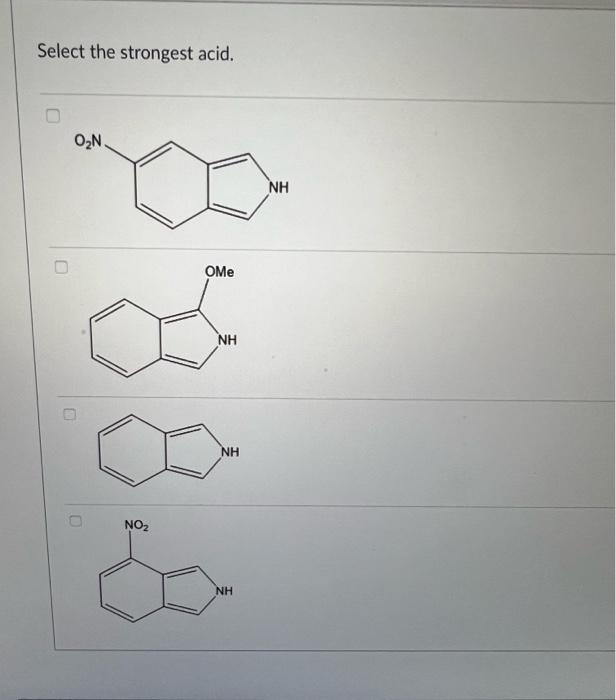
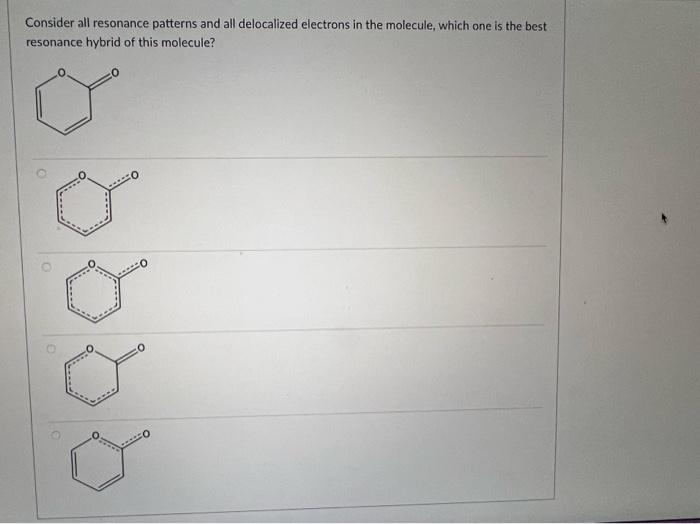


Select the strongest acid. Consider all resonance patterns and all delocalized electrons in the molecule, which one is the best resonance hybrid of this molecule? First consider the number of atoms and orbitals that participated in delocalization in the molecule below. If we use MO theory to study the delocalization, how many MO orbitals should we have? 2 3 5 4 All of the following are common organic solvents. Identify the polar molecules from the list below. 1. Acetone (CH3COCH3) 2. Ethanol (CH3CH2OH) 3. Methylene chloride (CH2Cl2) 4. Carbon tetrachloride (CCl4) 5. Toluene (C6H5CH3) 1,2,3 and 4 only 1 and 2 only 2 and 3 only 1,2 and 3 only
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


