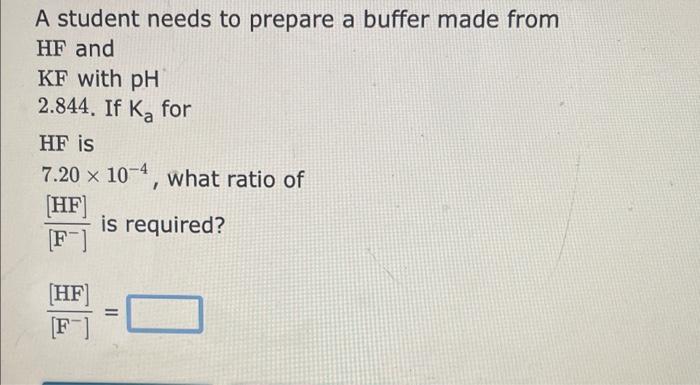
Question: i need help for the three questions A student needs to prepare a buffer made from HF and KF with pH 2.844. If Ka for
![for HF is 7.20104, what ratio of [F][HF]is required? [F][HF]= A student](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f83b9f9ead1_22366f83b9f2ff8d.jpg)
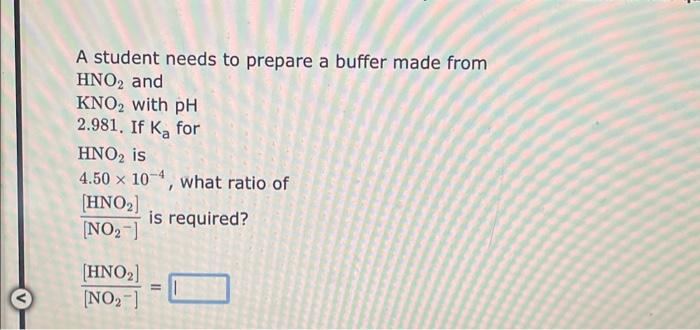
A student needs to prepare a buffer made from HF and KF with pH 2.844. If Ka for HF is 7.20104, what ratio of [F][HF]is required? [F][HF]= A student needs to prepare a buffer made from HNO2 and KNO2 with pH 2.981. If Ka for HNO2 is 4.50104, what ratio of [NO2][HNO2]is required? [NO2][HNO2]= A student needs to prepare a buffer made from CH3COOH and CH3COOK with pH 4.498. If Ka for CH3COOH is 1.80105, what ratio of [CH3COO][CH3COOH]is required? [CH3COO][CH3COOH]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


