Question: i need help for the three questions A buffer solution is made that is 0.328M in HF and 0.328M in NaF. (1) If Ka for
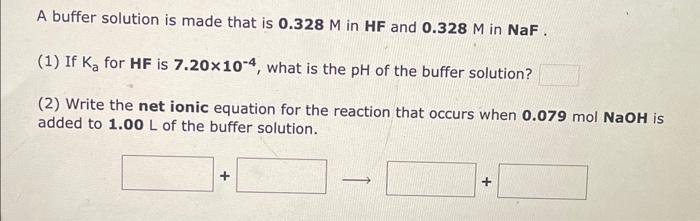


A buffer solution is made that is 0.328M in HF and 0.328M in NaF. (1) If Ka for HF is 7.20104, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.079molNaOH is added to 1.00L of the buffer solution. A buffer solution is made that is 0.354M in HNO2 and 0.354M in NaNO2. (1) If Ka for HNO2 is 4.50104, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.098molHNO3 is added to 1.00L of the buffer solution. Use H3O+instead of H+. A buffer solution is made that is 0.468M in CH3COOH and 0.468M in CH3COO. (1) If Ka for CH3COOH is 1.80105, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.100molHBr is added to 1.00L of the buffer solution. Use H3O+instead of H+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


