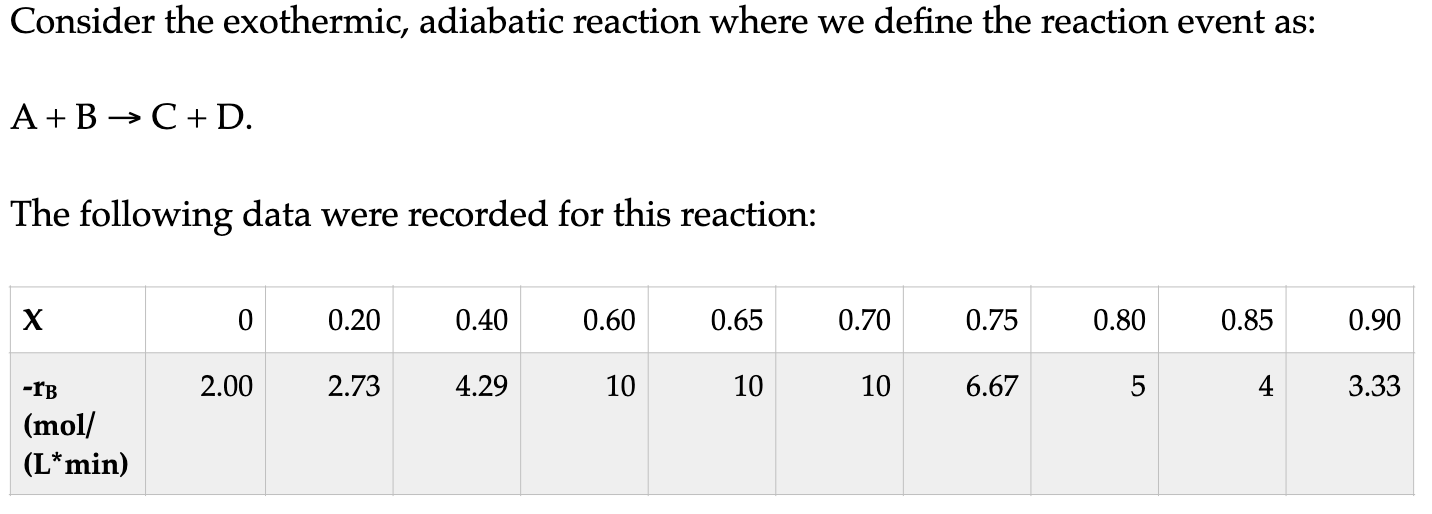
Question: I need help on c. d. and e. Thank you. Consider the exothermic, adiabatic reaction where we define the reaction event as: A+BC+D The following
I need help on c. d. and e.
Thank you.
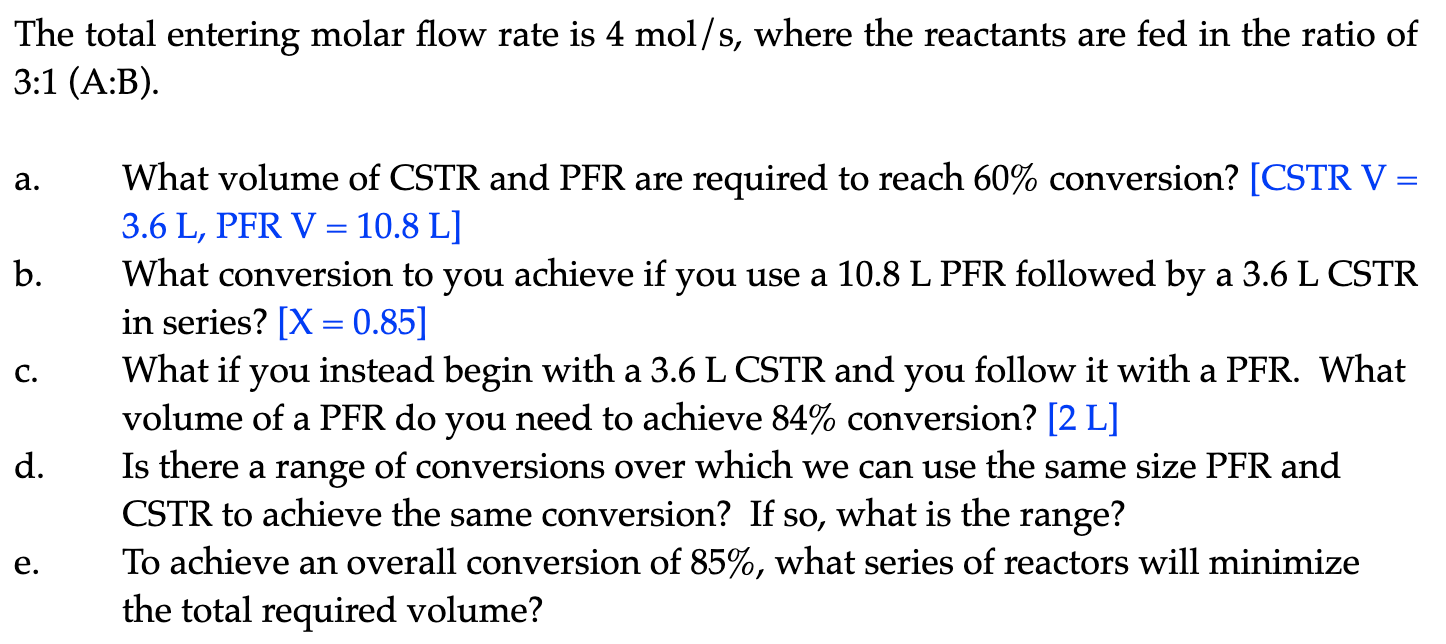
Consider the exothermic, adiabatic reaction where we define the reaction event as: A+BC+D The following data were recorded for this reaction: The total entering molar flow rate is 4mol/s, where the reactants are fed in the ratio of 3:1 (A:B). a. What volume of CSTR and PFR are required to reach 60% conversion? [CSTR V = 3.6 L, PFR V =10.8L] b. What conversion to you achieve if you use a 10.8 L PFR followed by a 3.6 L CSTR in series? [X=0.85] c. What if you instead begin with a 3.6L CSTR and you follow it with a PFR. What volume of a PFR do you need to achieve 84% conversion? [2 L] d. Is there a range of conversions over which we can use the same size PFR and CSTR to achieve the same conversion? If so, what is the range? e. To achieve an overall conversion of 85%, what series of reactors will minimize the total required volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


