Question: I need help understanding what I'm doing wrong. I errased the states of matter like the question asked, but I still need some help, I
I need help understanding what I'm doing wrong. I errased the states of matter like the question asked, but I still need some help, I don't know what is wrong. Thank you!
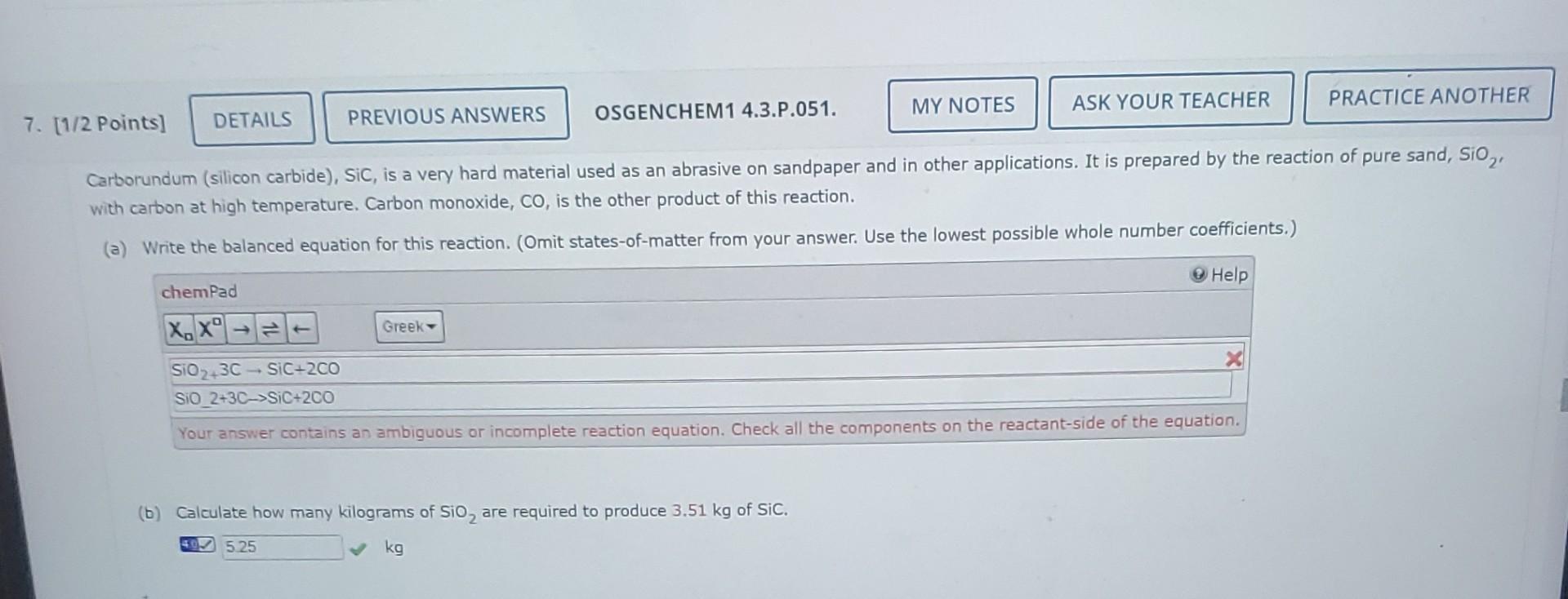
Carborundum (silicon carbide), SiC, is a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO 2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. (a) Write the balanced equation for this reaction. (Omit states-of-matter from your answer. Use the lowest possible whole number coefficients.) (b) Calculate how many kilograms of SiO2 are required to produce 3.51kg of SiC. kg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


