Question: i need help with part 2 please At high temperature, SO2 and NO2 react according to the equation below. Determine the equilibrium constant for this
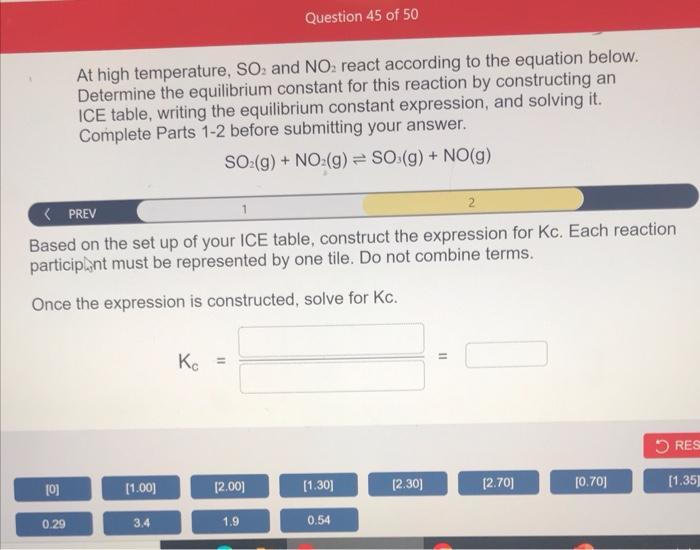
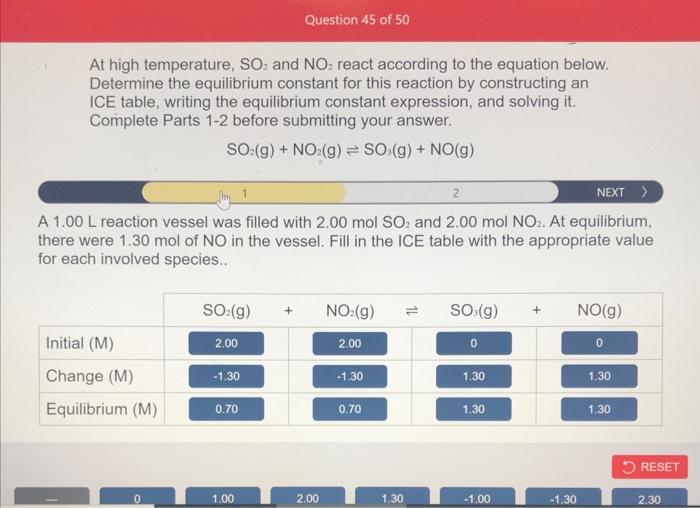
At high temperature, SO2 and NO2 react according to the equation below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. SO2(g)+NO2(g)SO3(g)+NO(g) Based on the set up of your ICE table, construct the expression for Kc. Each reaction particip int must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Kc. Kc== At high temperature, SO2 and NO2 react according to the equation below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 12 before submitting your answer. SO2(g)+NO2(g)SO3(g)+NO(g) A 1.00L reaction vessel was filled with 2.00molSO2 and 2.00molNO2. At equilibrium, there were 1.30mol of NO in the vessel. Fill in the ICE table with the appropriate value for each involved species
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


