Question: I need help with parts a, b and c please! 1) The density of nitrogen gas (N2,MW=28.02g/mol) inside of a gas cylinder is =118.6g/L at
I need help with parts a, b and c please! 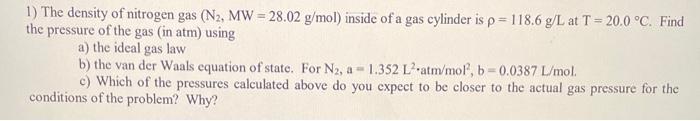
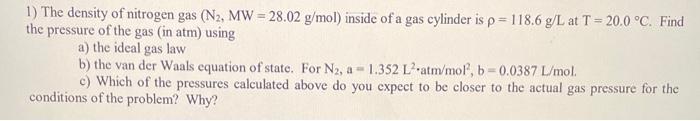
1) The density of nitrogen gas (N2,MW=28.02g/mol) inside of a gas cylinder is =118.6g/L at T=20.0C. Find the pressure of the gas (in atm) using a) the ideal gas law b) the van der Waals equation of state. For N2,a=1.352L2atm/mol2,b=0.0387L/mol. c) Which of the pressures calculated above do you expect to be closer to the actual gas pressure for the conditions of the problem? Why
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


