Question: I need help with step 2, please and thank you Methanol (CH3OH) burns with air. The product gas is analyzed and the laboratory report gives
I need help with step 2, please and thank you

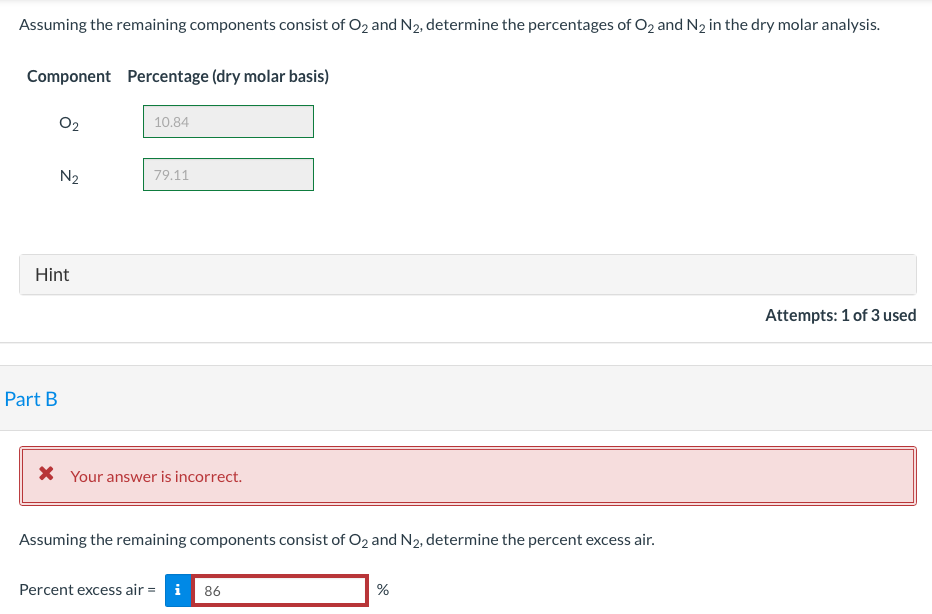
Methanol (CH3OH) burns with air. The product gas is analyzed and the laboratory report gives only the following percentages on a dry molar basis: 5% CO2, 3% CO, 0.84% CH3OH. Assuming the remaining components consist of O2 and N2, determine: (a) the percentages of O2 and N2 in the dry molar analysis. (b) the percent excess air. Assuming the remaining components consist of O2 and N2, determine the percentages of O2 and N2 in the dry molar analysis. Component Percentage (dry molar basis) 02 10.84 N2 79.11 Hint Attempts: 1 of 3 used Part B X Your answer is incorrect. Assuming the remaining components consist of O2 and N2, determine the percent excess air. Percent excess air = i 86 %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


