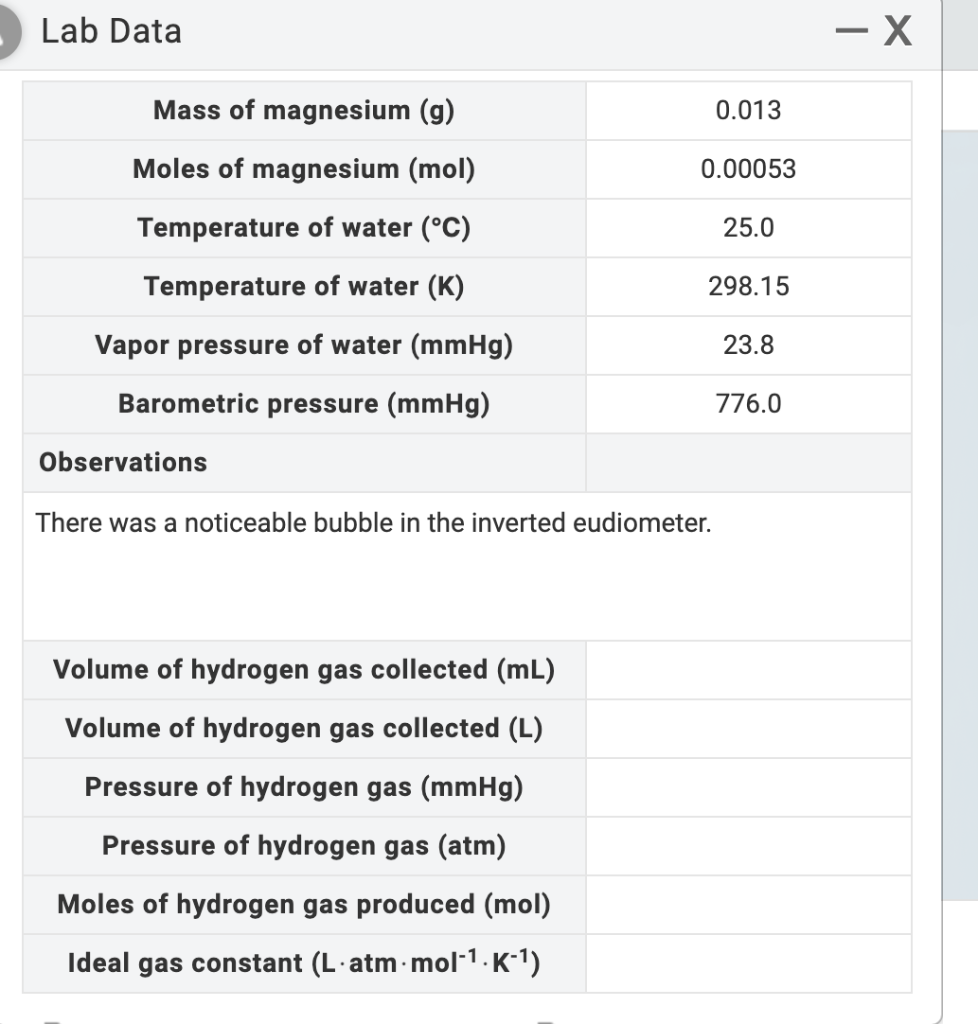
Question: I need help with the rest please Lab Data - X Mass of magnesium (g) 0.013 Moles of magnesium (mol) 0.00053 Temperature of water (C)
 I need help with the rest please
I need help with the rest please
Lab Data - X Mass of magnesium (g) 0.013 Moles of magnesium (mol) 0.00053 Temperature of water (C) 25.0 298.15 Temperature of water (K) Vapor pressure of water (mmHg) 23.8 Barometric pressure (mmHg) 776.0 Observations There was a noticeable bubble in the inverted eudiometer. Volume of hydrogen gas collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L.atm.mol-1.K-1) PHASE 6: Collecting hydrogen gas over water Complete the following steps: 12 Invert eudiometer into beaker of water 2 Observe reaction. Record your observations in Lab Data 13 3 At reaction's completion, equalize liquid heights. Zoom in on eudiometer and use up/down arrow to raise or lower eudiometer 14 4 Measure volume of hydrogen gas. Record in Lab Data DS RESET MY NOTES LAB DATA SHOW LABELS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


