Question: Whats in red is incorrect. please answer Lab Data -) Mass of magnesium (g) 0.017 Moles of magnesium (mol) 0.00070 Temperature of water (C) 25.0
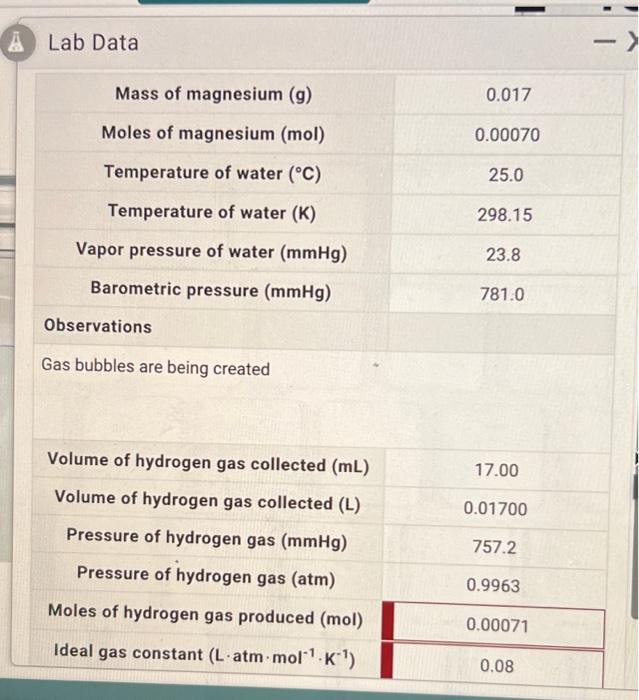
Lab Data -) Mass of magnesium (g) 0.017 Moles of magnesium (mol) 0.00070 Temperature of water (C) 25.0 Temperature of water (K) 298.15 Vapor pressure of water (mmHg) 23.8 Barometric pressure (mmHg) 781.0 Observations Gas bubbles are being created Volume of hydrogen gas collected (mL) 17.00 Volume of hydrogen gas collected (L) 0.01700 Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) 757.2 0.9963 Moles of hydrogen gas produced (mol) 0.00071 Ideal gas constant (L'atm.mol" K) 0.08
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


