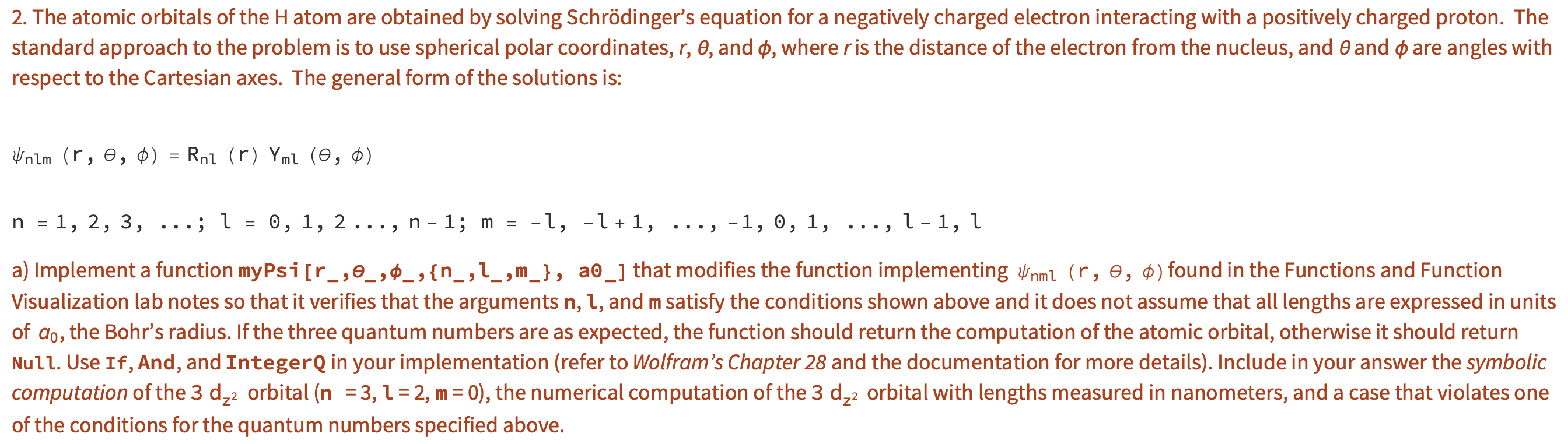
Question: I need help with this mathematic program problem. I don't know how to start the coding for it. 2. The atomic orbitals of the H
I need help with this mathematic program problem. I don't know how to start the coding for it.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


