Question: I need the answer as soon as possible 2. The following reaction takes place on a solid catalyst surface at 4.2 bar and 323 C.
I need the answer as soon as possible 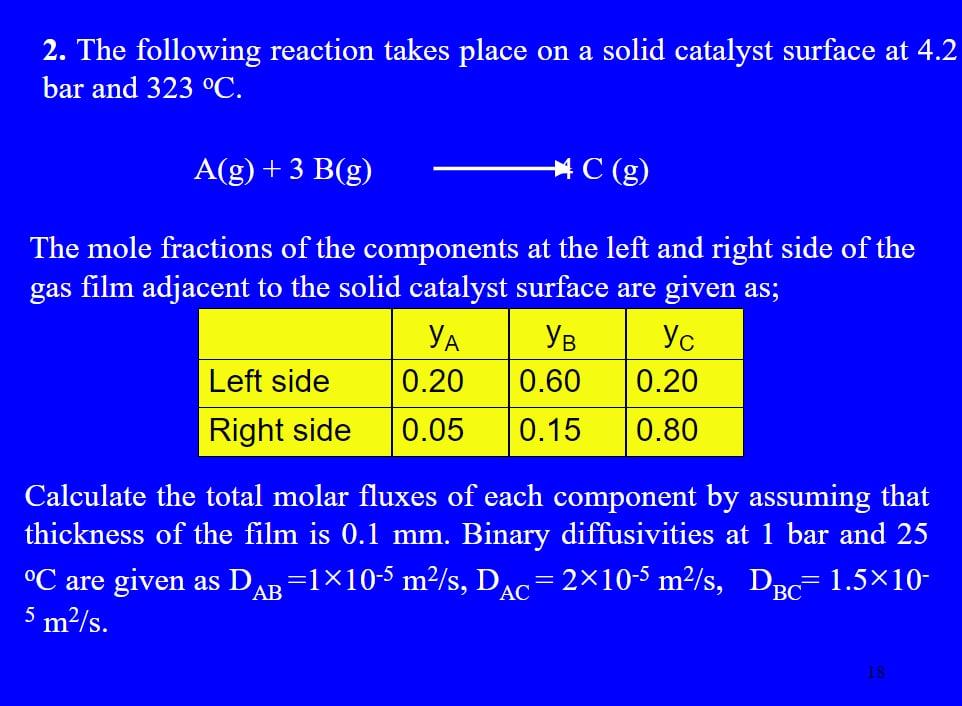
2. The following reaction takes place on a solid catalyst surface at 4.2 bar and 323 C. A(g) + 3 B(g) C (g) The mole fractions of the components at the left and right side of the gas film adjacent to the solid catalyst surface are given as; YB Left side 0.20 0.60 0.20 Right side 0.05 0.15 0.80 Calculate the total molar fluxes of each component by assuming that thickness of the film is 0.1 mm. Binary diffusivities at 1 bar and 25 C are given as DAB =1x10-5m2/s, Dac= 2x10-5 m2/s, DBC= 1.5X10- 5 m2/s. = AC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


