Question: What is the freezing point of a solution made by dissolving 10.35 g of naphthalene (C0Hg) in 100.0 g benzene (CH?)? The normal freezing

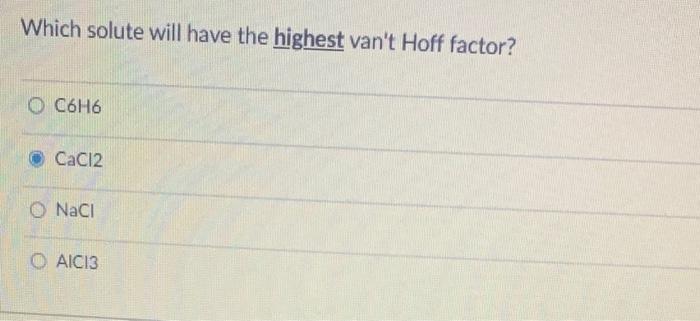
What is the freezing point of a solution made by dissolving 10.35 g of naphthalene (C0Hg) in 100.0 g benzene (CH?)? The normal freezing point of benzene is 5.5C and K f is 5.12C / m. O 0.0C O 14 06.9 O 3.4C Which solute will have the highest van't Hoff factor? O C6H6 CaCl2 O NaCl OAIC13
Step by Step Solution
★★★★★
3.41 Rating (148 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Freezing Point of Naphthalene Solution Given Mass of naphthalene CH1035 g Mass of benzene CH1000 g F... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


