Question: I need the answer as soon as possible Q.4. A chemical reaction AB is carried out in a closed vessel. The following data are taken
I need the answer as soon as possible 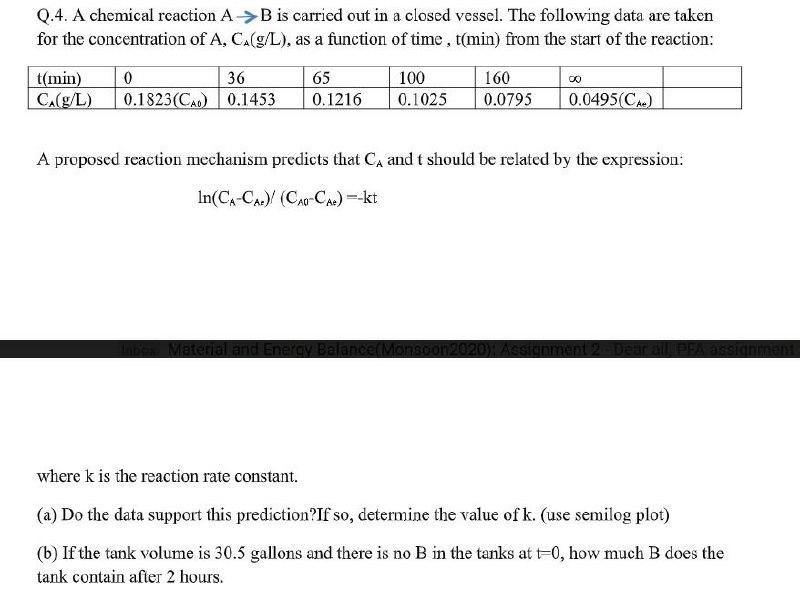
Q.4. A chemical reaction AB is carried out in a closed vessel. The following data are taken for the concentration of A, C.(g/L), as a function of time , t(min) from the start of the reaction: 00 t(min) CA(g/L) 0 36 | 0.1823(CAO) | 0.1453 65 0.1216 100 0.1025 160 0.0795 0.0495(CA) A proposed reaction mechanism predicts that CA and t should be related by the expression: In(CA-CA) (CAC-CA) --kt Material and Energy Balanc where k is the reaction rate constant. (a) Do the data support this prediction?If so, determine the value of k. (use semilog plot) (b) If the tank volume is 30.5 gallons and there is no B in the tanks at to, how much B does the tank contain after 2 hours
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


