Question: i posted this question for second time please answer it correctly thank you Determine the mass of precipitate (Ba3(PO4)2 produced when excess barium nitrate is
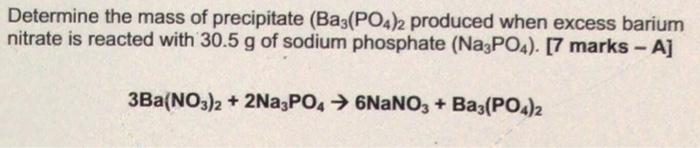 i posted this question for second time please answer it correctly thank you
i posted this question for second time please answer it correctly thank youDetermine the mass of precipitate (Ba3(PO4)2 produced when excess barium nitrate is reacted with 30.5 g of sodium phosphate (Na3PO4). [7 marks - A] 3Ba(NO3)2 + 2Na3PO4 NaNO3 + Baz(PO4)2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


