Question: i put 24.3 % as my answer and it was wrong Enter your answer in the provided box. Acetic acid is a weak acid that
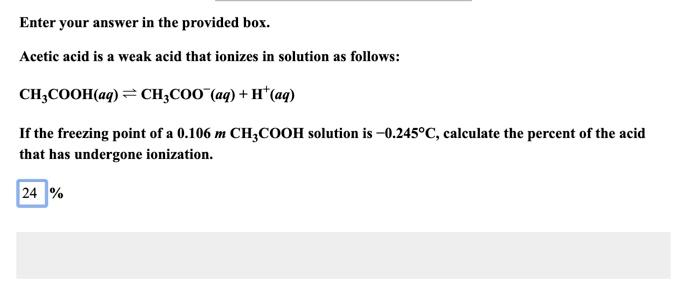 i put 24.3 % as my answer and it was wrong
i put 24.3 % as my answer and it was wrong
Enter your answer in the provided box. Acetic acid is a weak acid that ionizes in solution as follows: CH3COOH(aq)CH3COO(aq)+H+(aq) If the freezing point of a 0.106mCH3COOH solution is 0.245C, calculate the percent of the acid that has undergone ionization
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


