Question: I want the code using POLYMATH, thanks! Q (2). The irreversible gas phase elementary reaction: 2AB+2C is carried out in a membrane reactor in which
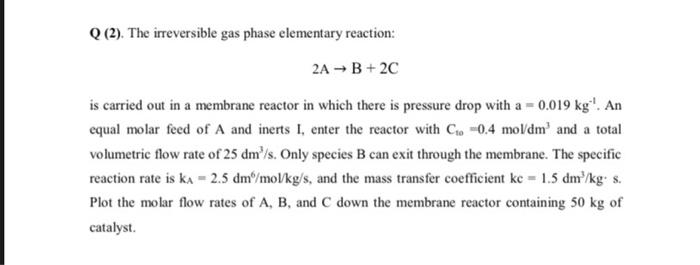
Q (2). The irreversible gas phase elementary reaction: 2AB+2C is carried out in a membrane reactor in which there is pressure drop with a =0.019kg1.An equal molar feed of A and inerts I, enter the reactor with C10=0.4mol/dm3 and a total volumetric flow rate of 25dm3/s. Only species B can exit through the membrane. The specific reaction rate is kA=2.5dm6/mol/kg/s, and the mass transfer coefficient kc=1.5dm3/kgs. Plot the molar flow rates of A,B, and C down the membrane reactor containing 50kg of catalyst
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


