Question: I would like an explaination on how they found the rate please Report Form: Experiment 2 Chemical Kinetics The lodine Clock Your original data, and
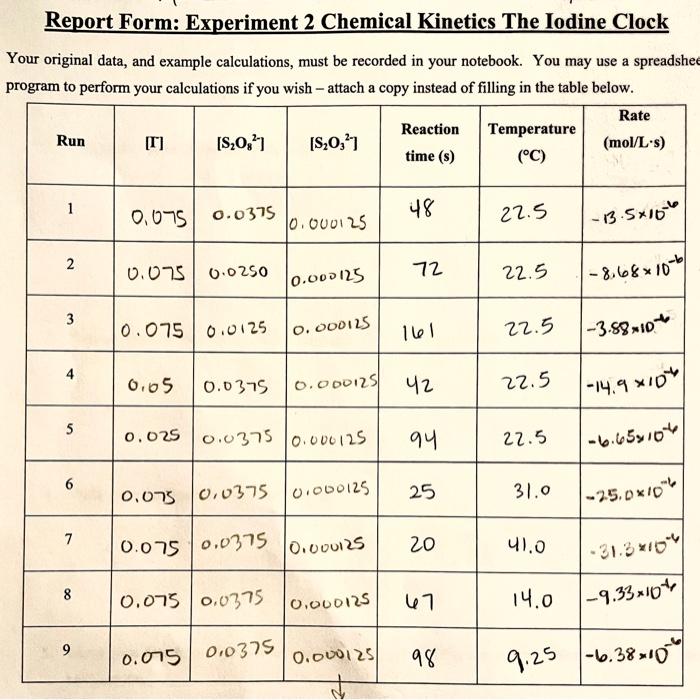
Report Form: Experiment 2 Chemical Kinetics The lodine Clock Your original data, and example calculations, must be recorded in your notebook. You may use a spreadshe program to perform your calculations if you wish - attach a copy instead of filling in the table below. Rate Reaction Temperature Run [T] [S,O) [S20,21 (mol/L's) time (s) (C) 1 48 22.5 0,075 0.0375 0.000125 1-13.5x16" 2 0.075 0.0250 72 0.000125 22.5 1-8.68x10-6 3 0.075 0.0125 o. 000125 161 22.5 |-3.8810 4 6.65 0.0375 0.000125 42 22.5 -14.9 X10 5 0.025 0.0375 0.000125 94 22.5 . 6 0,0775 0.0375 0.000125 25 31.0 -25.0*10** 7 0.075 0.0375 0.000125 20 41.0 8 0.075 0.0375 0.000125 67 14.0 1-9.33x104 9 0.075 0.0375 0.000125 98 9.25 -6.3810
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


