Question: Identify at least three errors in the given answers to the question, and explain how to correct the errors. 2. Ethanol (C2H5OH) melts at 114C
Identify at least three errors in the given answers to the question, and explain how to correct the errors.
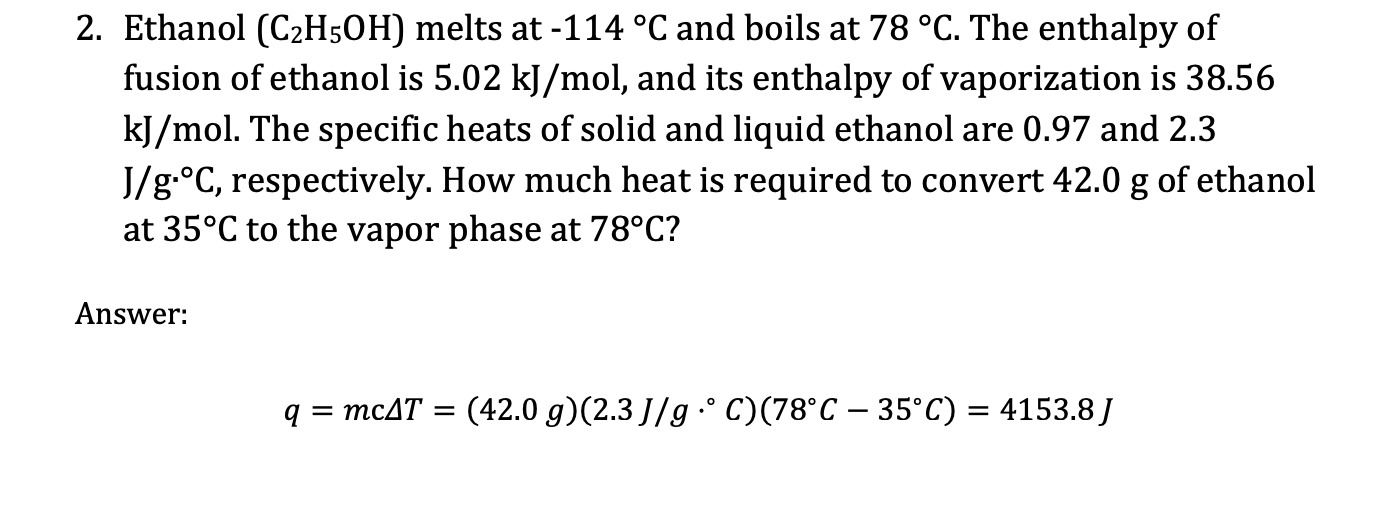
2. Ethanol (C2H5OH) melts at 114C and boils at 78C. The enthalpy of fusion of ethanol is 5.02kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/gC, respectively. How much heat is required to convert 42.0g of ethanol at 35C to the vapor phase at 78C ? Answer: q=mcT=(42.0g)(2.3J/gC)(78C35C)=4153.8J
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


