Question: Intermolecular Forces, Phase Changes, and Solutions Solved Problems (Extra Credit) At Attached is a pdf of solved problems that have a number of errors in
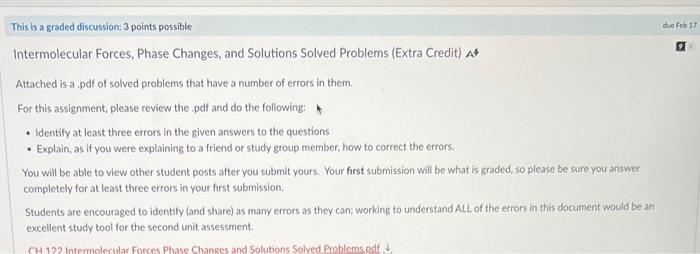
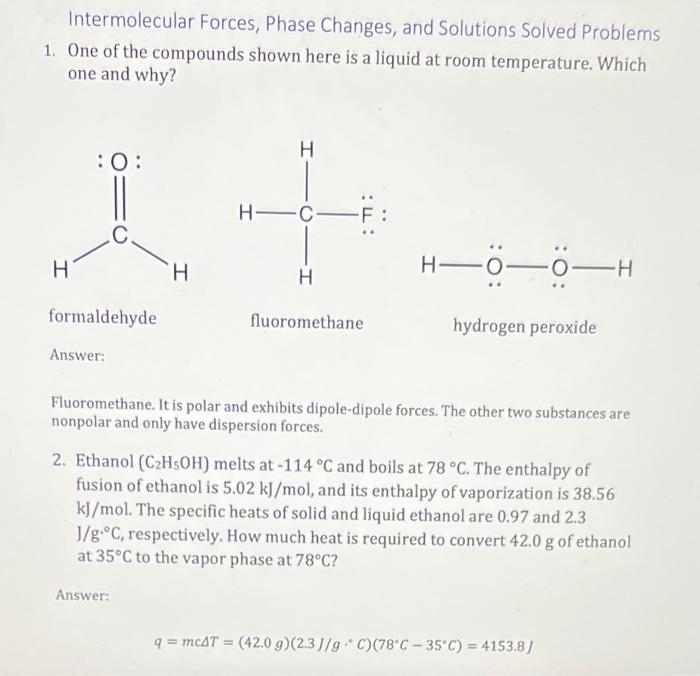

Intermolecular Forces, Phase Changes, and Solutions Solved Problems (Extra Credit) At Attached is a pdf of solved problems that have a number of errors in them. For this assignment, please review the pdf and do the following: - Identify at least three errors in the given answers to the questions - Explain, as if you were explaining to a friend or study group member, how to correct the errors. You will be able to view other student posts after you submit yours. Your first submission will be what is graded, so please be sure you answer completely for at least three errors in your first submission. Students are encouraged to identify (and share) as many errors as they can; working to understand ALL of the errors in this document would be an oxcellent study tool for the second unit assessment. Intermolecular Forces, Phase Changes, and Solutions Solved Problems 1. One of the compounds shown here is a liquid at room temperature. Which one and why? Fluoromethane. It is polar and exhibits dipole-dipole forces. The other two substances are nonpolar and only have dispersion forces. 2. Ethanol (C2H5OH) melts at 114C and boils at 78C. The enthalpy of fusion of ethanol is 5.02kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/gC, respectively. How much heat is required to convert 42.0g of ethanol at 35C to the vapor phase at 78C ? Answer: q=mcT=(42.0g)(2.3J/gC)(78C35C)=4153.8J Intermolecular Forces, Phase Changes, and Solutions Solved Problems 3. Cinnamon owes its flavor and odor to cinnamaldehyde (C9H8O). Determine the boiling point of a solution of 100.0mg of cinnamaldehyde dissolved in 1.00g of carbon tetrachloride. Answer: 100.0mg1000mg1g132.2g1mol=7.576104molC9H8O1.00g1000g1kg1kg1L=1.00103LCCl41.00103L7.576104mol=0.7576MTb=iKbm=(1)(5.03mC)(0.7576M)=3.81CTb=76.7C3.81C=72.9C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


