Question: Identify the net ionic equation for the reactions involved in this step of the conversion The cobalt carbonate preciptate is dissolved in excess sulfuric acid
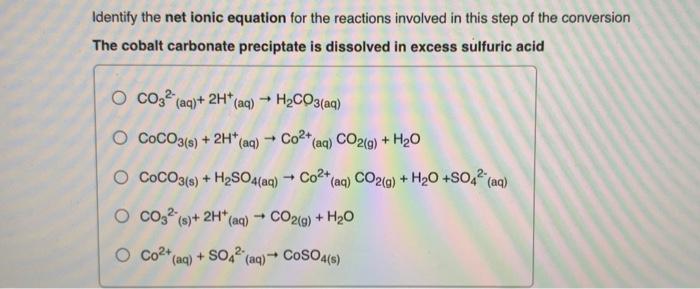
Identify the net ionic equation for the reactions involved in this step of the conversion The cobalt carbonate preciptate is dissolved in excess sulfuric acid O CO32 (aq)+ 2H+ (aq) H2CO3(aq) - - + - O COCO3(s) + 2H*(aq) Co2+ (aq) CO2(g) + H20 COCO3(s) + H2SO4(aq) + Co2+(aq) CO2(g) + H2O +SO42" (aq) O 0032 (9)+ 2H* * (aq) CO2(g) + H20 O Co2+ (aq) + SO42 (aq) + CoSO4(s) - +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


