Question: If a base ( represented as B: ) reacts with the most acidic proton on the given compound, where is the proper location
If a base represented as B: reacts with the most acidic proton on the given compound, where is the proper location for the end of
the curved arrow shown? Select the correct circled position for the terminus of the arrow.
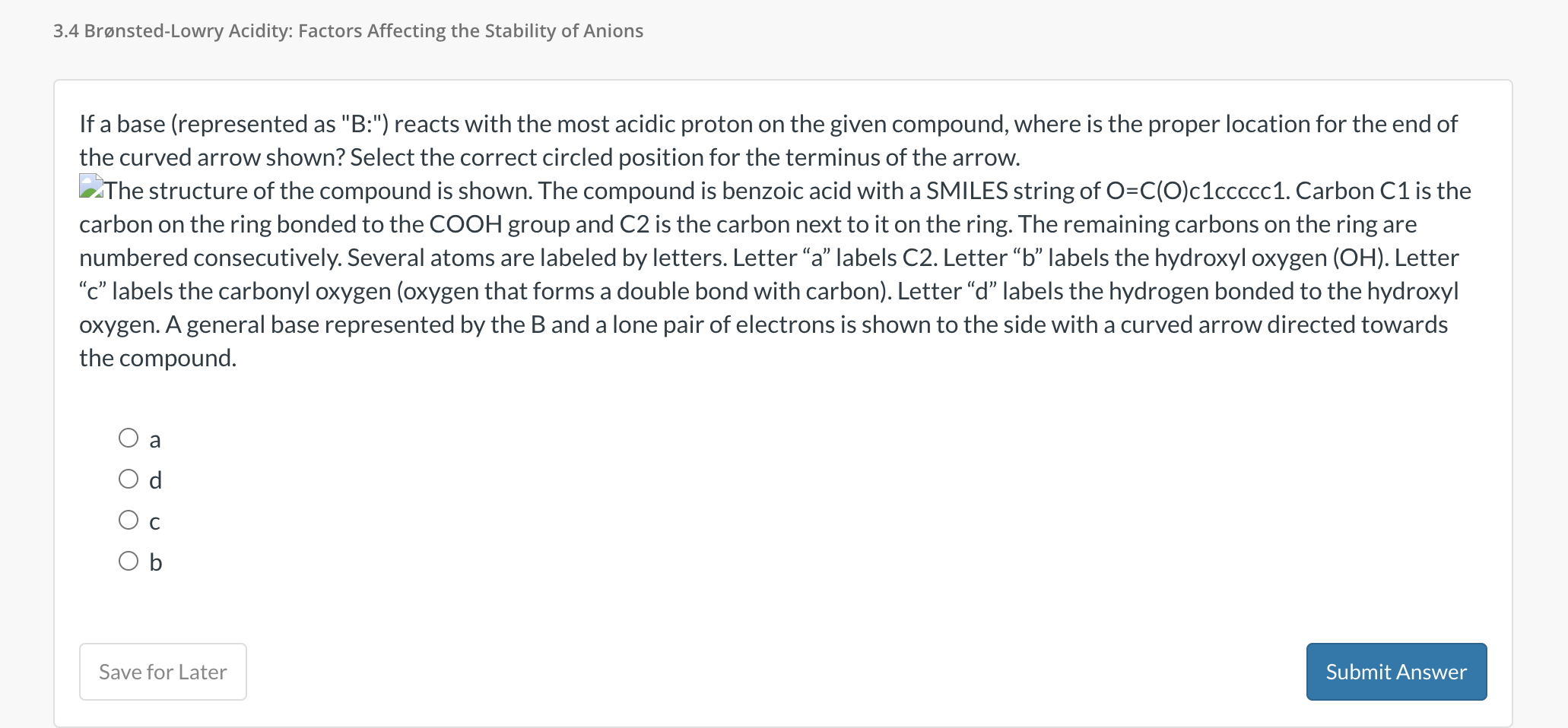
The structure of the compound is shown. The compound is benzoic acid with a SMILES string of Carbon is the
carbon on the ring bonded to the group and is the carbon next to it on the ring. The remaining carbons on the ring are
numbered consecutively. Several atoms are labeled by letters. Letter a labels C Letter b labels the hydroxyl oxygen OH Letter
c labels the carbonyl oxygen oxygen that forms a double bond with carbon Letter d labels the hydrogen bonded to the hydroxyl
oxygen. A general base represented by the B and a lone pair of electrons is shown to the side with a curved arrow directed towards
the compound.
a
d
c
b
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


