Question: If a buffer solution is 0.140 M in a weak acid (K, = 1.5 x 10 ) and 0.580 M in its conjugate base, what

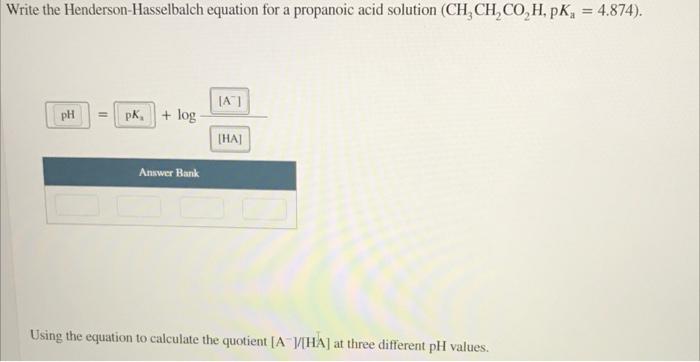

If a buffer solution is 0.140 M in a weak acid (K, = 1.5 x 10 ) and 0.580 M in its conjugate base, what is the pH? pH- TOOLS x10 Write the Henderson-Hasselbalch equation for a propanoic acid solution (CH CH,CO,H, PK, = 4.874). A) pH = pk + log THA Answer Bank Using the equation to calculate the quotient ( AHA) at three different pH values. Using the equation to calculate the quotient ( AHA) at three different pH values. pH = 4.191 0.2223 IA [HA] Incorrect pH = 4.874 IA 1 THA pH = 5.453 (A) 2.5468 THAI Income
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


