Question: If a buffer solution is 0.480 M in a weak acid (K, = 8.1 x 10-6) and 0.220 M in its conjugate base, what
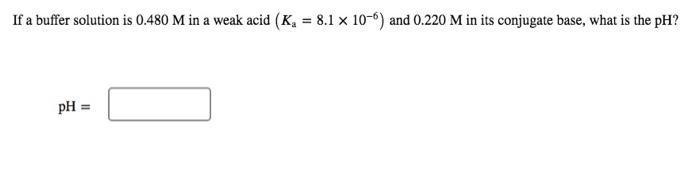
If a buffer solution is 0.480 M in a weak acid (K, = 8.1 x 10-6) and 0.220 M in its conjugate base, what is the pH? pH = %3!
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
63623a3237a62_236560.pdf
180 KBs PDF File
63623a3237a62_236560.docx
120 KBs Word File


