Question: If a buffer solution is 0.190 M in a weak acid (K, -6.4 x 10-) and 0.470 M in its conjugate base, what is the
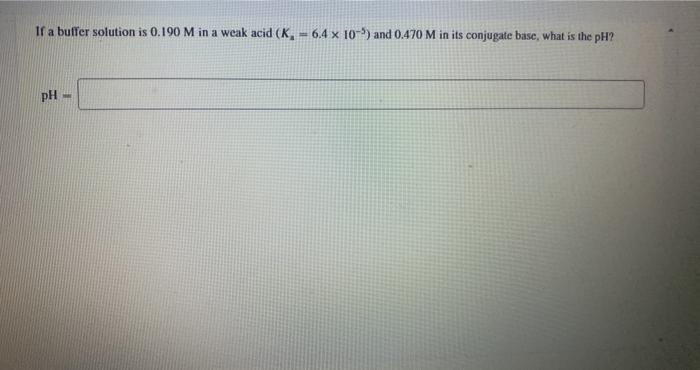
If a buffer solution is 0.190 M in a weak acid (K, -6.4 x 10-) and 0.470 M in its conjugate base, what is the pH? pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


