Question: If solid lithium hydrogen carbonate decomposes upon heating to form solid lithium oxide, gaseous carbon dioxide, and liquid water, which reaction below is the complete
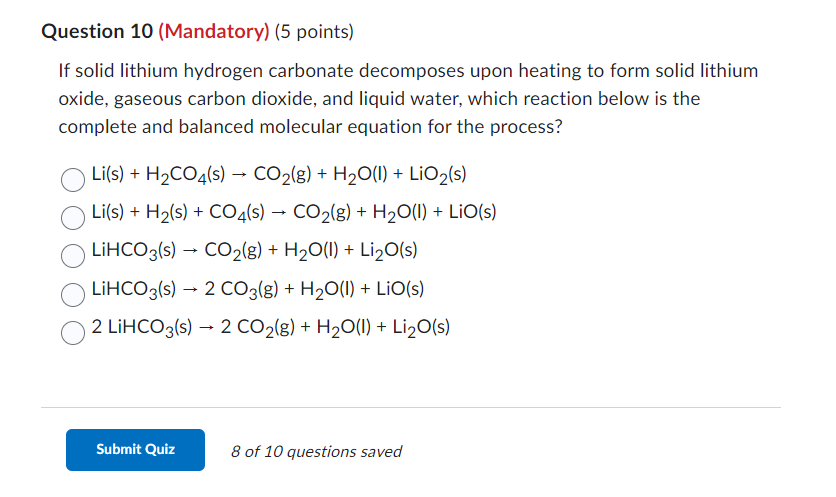

If solid lithium hydrogen carbonate decomposes upon heating to form solid lithium oxide, gaseous carbon dioxide, and liquid water, which reaction below is the complete and balanced molecular equation for the process? Li(s)+H2CO4(s)CO2(g)+H2O(I)+LiO2(s)Li(s)+H2(s)+CO4(s)CO2(g)+H2O(l)+LiO(s)LiHCO3(s)CO2(g)+H2O(l)+Li2O(s)LiHCO3(s)2CO3(g)+H2O(I)+LiO(s)2LiHCO3(s)2CO2(g)+H2O(I)+Li2O(s) 8 of 10 questions saved Choose all of the reactions below that correctly represent the balanced reaction of solid copper reacting with solid sulfur to form solid copper(I) sulfide. 2Cu(s)+S(s)Cu2S(s)S(s)+Cu(s)Cu2S(s)Cu(s)+S(s)Cu2S(s)16Cu(s)+S8(s)8Cu2S(s)S(s)+2Cu(s)Cu2S(s)S(s)+16Cu(s)8Cu2S(s)Cu(s)+S(s)CuS(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


