Question: If you cannot answer both questions, please answer the first one (question 15) 15. Establish the theoretical initial rate laws for CO2 decomposition for the
If you cannot answer both questions, please answer the first one (question 15)
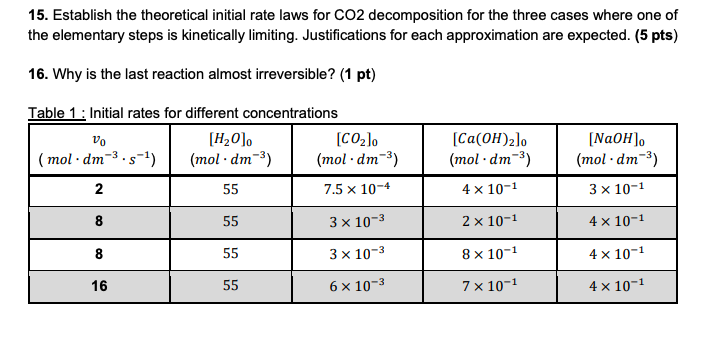
15. Establish the theoretical initial rate laws for CO2 decomposition for the three cases where one of the elementary steps is kinetically limiting. Justifications for each approximation are expected. (5 pts) 16. Why is the last reaction almost irreversible? (1 pt) Table 1: Initial rates for different concentrations VO [H0]. (CO2. (mol.dm-3.5-) (mol.dm-3) (mol.dm-3) 2 55 7.5 x 10-4 [Ca(OH)2lo (mol.dm-3) [NaOH). (mol.dm-3) 4 x 10-1 3 x 10-1 8 55 3 x 10-3 2 x 10-1 4 x 10-1 8 55 3 x 10-3 8 x 10-1 4 x 10-1 16 55 6 x 10-3 7 x 10-1 4 x 10-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


