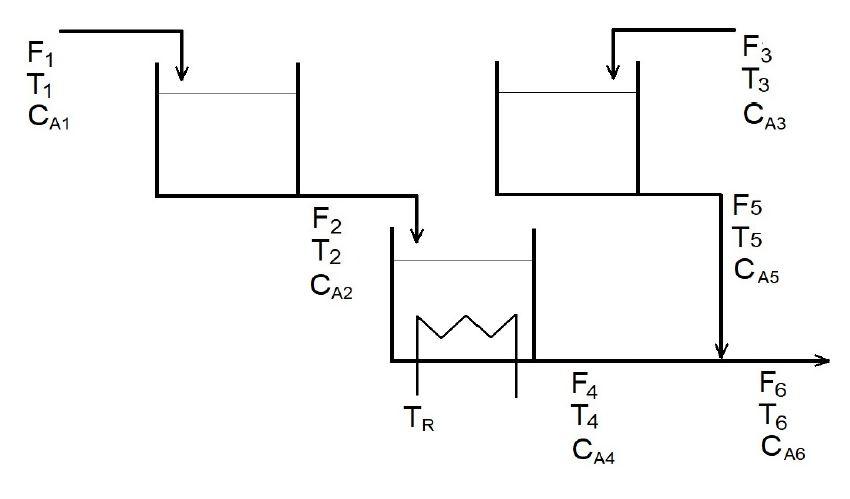
Question: (IF YOU DON'T PUT THE FULL ANSWER PLEASE DO NOT ANSWER) Two stirred tanks and a CSTR reactor with volumes equal to 13.26 ft3 each
(IF YOU DON'T PUT THE FULL ANSWER PLEASE DO NOT ANSWER) Two stirred tanks and a CSTR reactor with volumes equal to 13.26 ft3 each are used to produce compound B in an exothermic reaction A B with a final CA6 concentration of 0.08023 lbmols/ft3 at a constant flow rate of F1 and F3 = 1.3364 ft3/min, density of 55.0 lbm/ft3 and Cp = 0.88 Btu/lb.oF. The process is initially operating with a concentration of CA1 equal to 0.8983 lbmol/ft3 and CA3 = 0. Further system data is given below. Using Transfer Functions to get the results:
(a) If the temperature of the refrigerant TR is increased by 10 R what will be the new outlet temperature T6.
(b) If the inlet temperature T1 is suddenly increased from 578 to 588 R, how long will it take for the inlet temperature to output T5 change 10 R?
T1 = 578 R
c1 = 0,8983 lbmols/ft3 Tc = 560 R F1 = 1,3364 ft3/min c4 = 0,08023 lbmol/ft3 T4 = 690 R A = 36 ft2 V = 13,26 ft3 Ro = 55,0 lbm/ft3 Cp = 0,88 Btu/lb.F R = 1,987 Btu/lbmol.R HR = 12000 Btu/lbmol U = 75 Btu/h.ft2.F ko = 833000000 min-1 E = 27820 Btu/lbmol
F1 T1 F3 T CA1 F5 LE T5 F2 2 CAz CA5 F4 T4 4 F6 TR 6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


