Question: im having problems understanding the math or what to do to get started, help Benzoic Acid A. Preparation of Experimental Apparatus and Freezing Point Determination
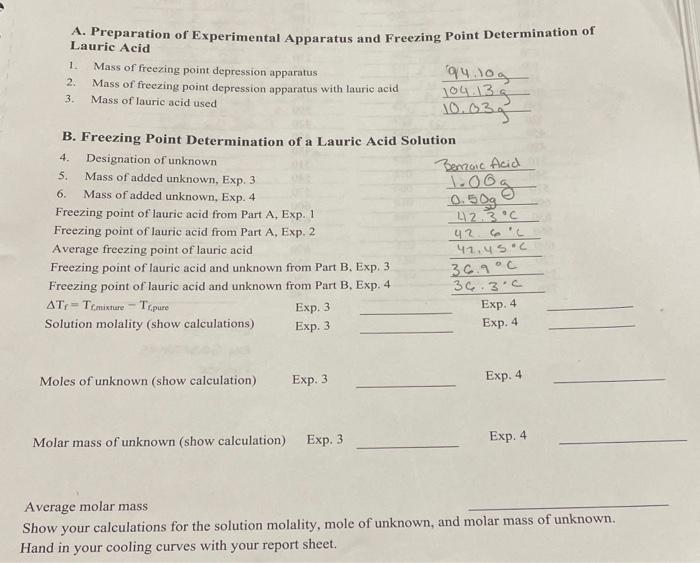
Benzoic Acid A. Preparation of Experimental Apparatus and Freezing Point Determination of Lauric Acid 1. Mass of freezing point depression apparatus q4.log 2. Mass of freezing point depression apparatus with lauric acid 104.135 3. Mass of lauric acid used 10.03g B. Freezing Point Determination of a Lauric Acid Solution 4. Designation of unknown 5. Mass of added unknown, Exp. 3 100g 6. Mass of added unknown. Exp. 4 0.5 og Freezing point of lauric acid from Part A, Exp. 1 42.3 C Freezing point of lauric acid from Part A, Exp. 2 Average freezing point of lauric acid Freezing point of lauric acid and unknown from Part B. Exp. 3 Freezing point of lauric acid and unknown from Part B. Exp. 4 36.3.0 AT, -Te mixture - Ti.pure Solution molality (show calculations) Exp. 3 uz 42.4s.c 36.9C Exp. 3 Exp. 4 Exp. 4 Moles of unknown (show calculation) Exp. 3 Exp. 4 Molar mass of unknown (show calculation) Exp. 3 Exp. 4 Average molar mass Show your calculations for the solution molality, mole of unknown, and molar mass of unknown. Hand in your cooling curves with your report sheet
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


