Question: I'm totally lost on these problems. 7 1 point If 4.00 x 102) atoms of calcium are contained in 356.5 ml. of water, what is
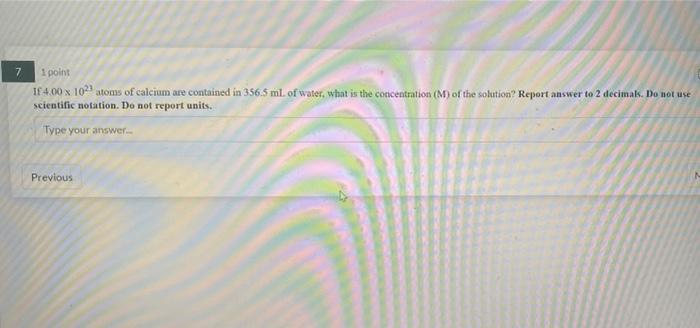
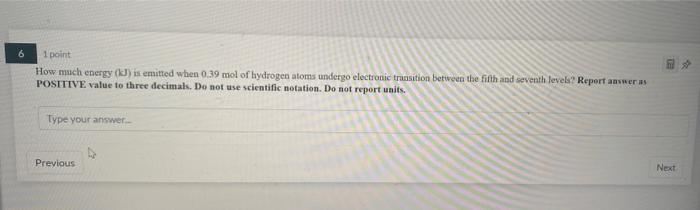
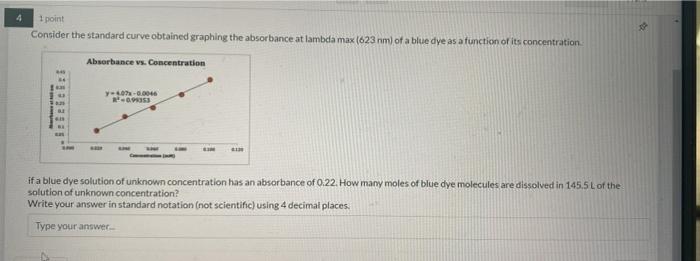
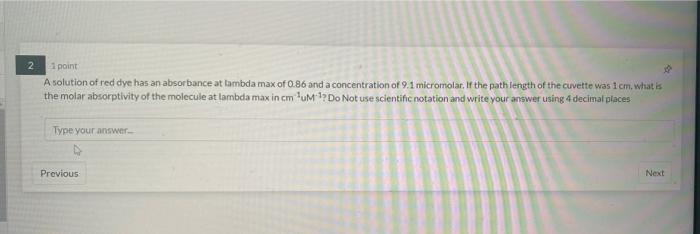
7 1 point If 4.00 x 102) atoms of calcium are contained in 356.5 ml. of water, what is the concentration (M) of the solution? Report answer to 2 decimals. Do not use scientific notation. Do not report units. Type your answer Previous 1 point How much energy (J) is emitted when 0.39 mol of hydrogen atoms undergo electronic transition between the fifth and seventh levels? Report answer as POSITIVE value to three decimals. Do not use scientific notation. Do not report units. Type your answer Previous Next 1 point Consider the standard curve obtained graphing the absorbance at lambda max (623 nm) of a blue dye as a function of its concentration Absorbance vs. Concentration 1 02-0.0046 R-005 su if a blue dye solution of unknown concentration has an absorbance of 0.22. How many moles of blue dye molecules are dissolved in 145.5 L of the solution of unknown concentration? Write your answer in standard notation (not scientific) using 4 decimal places. Type your answer 2 2 1 point A solution of red dye has an absorbance at tambda max of 0.86 and a concentration of 9.1 micromolar. If the path length of the cuvette was 1 cm, what is the molar absorptivity of the molecule at lambda max in cm um ? Do Not use scientific notation and write your answer using 4decimal places Type your answer Previous Next
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


